Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea
- PMID: 26280222
- PMCID: PMC4626298
- DOI: 10.1038/labinvest.2015.113
Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea
Abstract
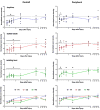
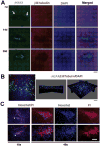
Although sensory reinnervation occurs after injury in the peripheral nervous system, poor reinnervation in the elderly and those with diabetes often leads to pathology. Here we quantify sub-basal axon density in the central and peripheral mouse cornea over time after three different types of injury. The mouse cornea is highly innervated with a dense array of sub-basal nerves that form a spiral called the vortex at the corneal center or apex; these nerves are readily detected within flat mounted corneas. After anesthesia, corneal epithelial cells were removed using either a dulled blade or a rotating burr within an area demarcated centrally with a 1.5 mm trephine. A third wound type, superficial trephination, involved demarcating the area with the 1.5 mm trephine but not removing cells. By 7 days after superficial trephination, sub-basal axon density returns to control levels; by 28 days the vortex reforms. Although axon density is similar to control 14 days after dulled blade and rotating burr wounding, defects in axon morphology at the corneal apex remain. After 14 days, axons retract from the center leaving the sub-basal axon density reduced by 37.2 and 36.8% at 28 days after dulled blade and rotating burr wounding, respectively, compared with control. Assessment of inflammation using flow cytometry shows that persistent inflammation is not a factor in the incomplete reinnervation. Expression of mRNAs encoding 22 regeneration-associated genes involved in axon targeting assessed by QPCR reveals that netrin-1 and ephrin signaling are altered after wounding. Subpopulations of corneal epithelial basal cells at the corneal apex stop expressing ki67 as early as 7 days after injury and by 14 and 28 days after wounding, many of these basal cells undergo apoptosis and die. Although sub-basal axons are restored to their normal density and morphology after superficial trephination, sub-basal axon recovery is partial after debridement wounds. The increase in corneal epithelial basal cell apoptosis at the apex observed at 14 days after corneal debridement may destabilize newly reinnervated sub-basal axons and lead to their retraction toward the periphery.
Figures




Similar articles
-
Molecular basis of Mitomycin C enhanced corneal sensory nerve repair after debridement wounding.Sci Rep. 2018 Nov 16;8(1):16960. doi: 10.1038/s41598-018-35090-3. Sci Rep. 2018. PMID: 30446696 Free PMC article.
-
Cytokine deposition alters leukocyte morphology and initial recruitment of monocytes and γδT cells after corneal injury.Invest Ophthalmol Vis Sci. 2014 Apr 28;55(4):2757-65. doi: 10.1167/iovs.13-13557. Invest Ophthalmol Vis Sci. 2014. PMID: 24677104 Free PMC article.
-
Topical Mitomycin-C enhances subbasal nerve regeneration and reduces erosion frequency in the debridement wounded mouse cornea.Exp Eye Res. 2016 May;146:361-369. doi: 10.1016/j.exer.2015.08.023. Epub 2015 Aug 30. Exp Eye Res. 2016. PMID: 26332224 Free PMC article.
-
Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves.Glia. 2017 Jun;65(6):851-863. doi: 10.1002/glia.23102. Epub 2016 Nov 23. Glia. 2017. PMID: 27878997 Free PMC article. Review.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
Cited by
-
The impact of euthanasia and enucleation on mouse corneal epithelial axon density and nerve terminal morphology.Ocul Surf. 2020 Oct;18(4):821-828. doi: 10.1016/j.jtos.2020.07.021. Epub 2020 Aug 13. Ocul Surf. 2020. PMID: 32798735 Free PMC article.
-
ROCK Inhibitor Enhances Neurite Outgrowth In Vitro and Corneal Sensory Nerve Reinnervation In Vivo.Invest Ophthalmol Vis Sci. 2024 Oct 1;65(12):31. doi: 10.1167/iovs.65.12.31. Invest Ophthalmol Vis Sci. 2024. PMID: 39436373 Free PMC article.
-
Limited versus total epithelial debridement ocular surface injury: Live fluorescence imaging of hemangiogenesis and lymphangiogenesis in Prox1-GFP/Flk1::Myr-mCherry mice.Biochim Biophys Acta. 2016 Oct;1860(10):2148-56. doi: 10.1016/j.bbagen.2016.05.027. Epub 2016 May 24. Biochim Biophys Acta. 2016. PMID: 27233452 Free PMC article.
-
Expression of axon guidance ligands and their receptors in the cornea and trigeminal ganglia and their recovery after corneal epithelium injury.Exp Eye Res. 2022 Jun;219:109054. doi: 10.1016/j.exer.2022.109054. Epub 2022 Apr 12. Exp Eye Res. 2022. PMID: 35427568 Free PMC article.
-
Molecular basis of Mitomycin C enhanced corneal sensory nerve repair after debridement wounding.Sci Rep. 2018 Nov 16;8(1):16960. doi: 10.1038/s41598-018-35090-3. Sci Rep. 2018. PMID: 30446696 Free PMC article.
References
-
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–42. - PubMed
-
- Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46:4485–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

