Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis
- PMID: 26280336
- PMCID: PMC4607316
- DOI: 10.1038/nature14975
Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis
Abstract
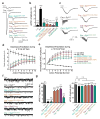
Synaptotagmin-1 and neuronal SNARE proteins have central roles in evoked synchronous neurotransmitter release; however, it is unknown how they cooperate to trigger synaptic vesicle fusion. Here we report atomic-resolution crystal structures of Ca(2+)- and Mg(2+)-bound complexes between synaptotagmin-1 and the neuronal SNARE complex, one of which was determined with diffraction data from an X-ray free-electron laser, leading to an atomic-resolution structure with accurate rotamer assignments for many side chains. The structures reveal several interfaces, including a large, specific, Ca(2+)-independent and conserved interface. Tests of this interface by mutagenesis suggest that it is essential for Ca(2+)-triggered neurotransmitter release in mouse hippocampal neuronal synapses and for Ca(2+)-triggered vesicle fusion in a reconstituted system. We propose that this interface forms before Ca(2+) triggering, moves en bloc as Ca(2+) influx promotes the interactions between synaptotagmin-1 and the plasma membrane, and consequently remodels the membrane to promote fusion, possibly in conjunction with other interfaces.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








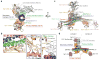



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 GM102520/GM/NIGMS NIH HHS/United States
- R01 GM077071/GM/NIGMS NIH HHS/United States
- GM095887/GM/NIGMS NIH HHS/United States
- R37 MH063105/MH/NIMH NIH HHS/United States
- R37MH63105/MH/NIMH NIH HHS/United States
- P41GM103393/GM/NIGMS NIH HHS/United States
- GM102520/GM/NIGMS NIH HHS/United States
- P41 GM103403/GM/NIGMS NIH HHS/United States
- MH086403/MH/NIMH NIH HHS/United States
- P50 MH086403/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM095887/GM/NIGMS NIH HHS/United States
- P41 GM103393/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

