Radiation-Induced Glycogen Accumulation Detected by Single Cell Raman Spectroscopy Is Associated with Radioresistance that Can Be Reversed by Metformin
- PMID: 26280348
- PMCID: PMC4539228
- DOI: 10.1371/journal.pone.0135356
Radiation-Induced Glycogen Accumulation Detected by Single Cell Raman Spectroscopy Is Associated with Radioresistance that Can Be Reversed by Metformin
Abstract
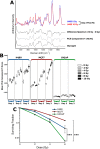
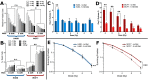
Altered cellular metabolism is a hallmark of tumor cells and contributes to a host of properties associated with resistance to radiotherapy. Detection of radiation-induced biochemical changes can reveal unique metabolic pathways affecting radiosensitivity that may serve as attractive therapeutic targets. Using clinically relevant doses of radiation, we performed label-free single cell Raman spectroscopy on a series of human cancer cell lines and detected radiation-induced accumulation of intracellular glycogen. The increase in glycogen post-irradiation was highest in lung (H460) and breast (MCF7) tumor cells compared to prostate (LNCaP) tumor cells. In response to radiation, the appearance of this glycogen signature correlated with radiation resistance. Moreover, the buildup of glycogen was linked to the phosphorylation of GSK-3β, a canonical modulator of cell survival following radiation exposure and a key regulator of glycogen metabolism. When MCF7 cells were irradiated in the presence of the anti-diabetic drug metformin, there was a significant decrease in the amount of radiation-induced glycogen. The suppression of glycogen by metformin following radiation was associated with increased radiosensitivity. In contrast to MCF7 cells, metformin had minimal effects on both the level of glycogen in H460 cells following radiation and radiosensitivity. Our data demonstrate a novel approach of spectral monitoring by Raman spectroscopy to assess changes in the levels of intracellular glycogen as a potential marker and resistance mechanism to radiation therapy.
Conflict of interest statement
Figures



References
-
- Sanli T, Rashid A, Liu C, Harding S, Bristow RG, Cutz JC, et al. Ionizing radiation activates AMP-activated kinase (AMPK): a target for radiosensitization of human cancer cells. International journal of radiation oncology, biology, physics. 2010;78(1):221–9. 10.1016/j.ijrobp.2010.03.005 - DOI - PubMed
-
- Shimura T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. Journal of radiation research. 2011;52(5):539–44. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

