Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes
- PMID: 26280654
- PMCID: PMC4632072
- DOI: 10.1038/oncsis.2015.21
Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes
Abstract
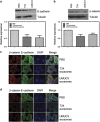
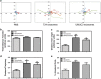
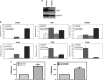
Bladder cancer, the fourth most common noncutaneous malignancy in the United States, is characterized by high recurrence rate, with a subset of these cancers progressing to a deadly muscle invasive form of disease. Exosomes are small secreted vesicles that contain proteins, mRNA and miRNA, thus potentially modulating signaling pathways in recipient cells. Epithelial-to-mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal stem cells. EMT has been implicated in the initiation of metastasis for cancer progression. We investigated the ability of bladder cancer-shed exosomes to induce EMT in urothelial cells. Exosomes were isolated by ultracentrifugation from T24 or UMUC3 invasive bladder cancer cell conditioned media or from patient urine or bladder barbotage samples. Exosomes were then added to the urothelial cells and EMT was assessed. Urothelial cells treated with bladder cancer exosomes showed an increased expression in several mesenchymal markers, including α-smooth muscle actin, S100A4 and snail, as compared with phosphate-buffered saline (PBS)-treated cells. Moreover, treatment of urothelial cells with bladder cancer exosomes resulted in decreased expression of epithelial markers E-cadherin and β-catenin, as compared with the control, PBS-treated cells. Bladder cancer exosomes also increased the migration and invasion of urothelial cells, and this was blocked by heparin pretreatment. We further showed that exosomes isolated from patient urine and bladder barbotage samples were able to induce the expression of several mesenchymal markers in recipient urothelial cells. In conclusion, the research presented here represents both a new insight into the role of exosomes in transition of bladder cancer into invasive disease, as well as an introduction to a new platform for exosome research in urothelial cells.
Figures



References
-
- 1Resnick MJ, Chang SS. Optimizing outcomes for octogenarians with invasive bladder cancer: one size does not fit all. Urol Oncol 2013; 31: 1–4. - PubMed
-
- 3Messing EM. Urothelial tumors of the urinary tract. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ (eds). Campbell's Urology, 8th edn. Saunders: Philadelphia, PA, USA, 2002, pp 2750–2751.
-
- 4Messing EM. Urothelial tumors of the bladder. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds). Campbell-Walsh's Urology. Saunders: Philadelphia, PA, USA, 2007, pp 17–2430.
-
- 5Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113(Pt 19): 3365–3374. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

