Mathematical Model of Ammonia Handling in the Rat Renal Medulla
- PMID: 26280830
- PMCID: PMC4539222
- DOI: 10.1371/journal.pone.0134477
Mathematical Model of Ammonia Handling in the Rat Renal Medulla
Abstract
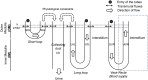
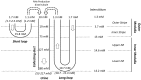
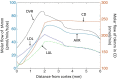
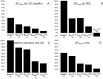
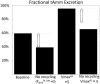
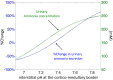
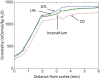
The kidney is one of the main organs that produces ammonia and release it into the circulation. Under normal conditions, between 30 and 50% of the ammonia produced in the kidney is excreted in the urine, the rest being absorbed into the systemic circulation via the renal vein. In acidosis and in some pathological conditions, the proportion of urinary excretion can increase to 70% of the ammonia produced in the kidney. Mechanisms regulating the balance between urinary excretion and renal vein release are not fully understood. We developed a mathematical model that reflects current thinking about renal ammonia handling in order to investigate the role of each tubular segment and identify some of the components which might control this balance. The model treats the movements of water, sodium chloride, urea, NH3 and [Formula: see text], and non-reabsorbable solute in an idealized renal medulla of the rat at steady state. A parameter study was performed to identify the transport parameters and microenvironmental conditions that most affect the rate of urinary ammonia excretion. Our results suggest that urinary ammonia excretion is mainly determined by those parameters that affect ammonia recycling in the loops of Henle. In particular, our results suggest a critical role for interstitial pH in the outer medulla and for luminal pH along the inner medullary collecting ducts.
Conflict of interest statement
Figures



Similar articles
-
Maximum urine concentrating capability in a mathematical model of the inner medulla of the rat kidney.Bull Math Biol. 2010 Feb;72(2):314-39. doi: 10.1007/s11538-009-9448-0. Bull Math Biol. 2010. PMID: 19915926 Free PMC article.
-
Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct.J Clin Invest. 1982 Jul;70(1):1-12. doi: 10.1172/jci110581. J Clin Invest. 1982. PMID: 7085880 Free PMC article.
-
Transepithelial ammonia concentration gradients in inner medulla of the rat.Am J Physiol. 1987 Mar;252(3 Pt 2):F491-500. doi: 10.1152/ajprenal.1987.252.3.F491. Am J Physiol. 1987. PMID: 3826390
-
Ammonium transport by the loop of Henle.Miner Electrolyte Metab. 1990;16(5):291-8. Miner Electrolyte Metab. 1990. PMID: 2283992 Review.
-
Role of the thick ascending limb and inner medullary collecting duct in the regulation of urinary acidification.Semin Nephrol. 1991 Mar;11(2):120-8. Semin Nephrol. 1991. PMID: 2034922 Review. No abstract available.
Cited by
-
Ammonia Transporters and Their Role in Acid-Base Balance.Physiol Rev. 2017 Apr;97(2):465-494. doi: 10.1152/physrev.00011.2016. Physiol Rev. 2017. PMID: 28151423 Free PMC article. Review.
-
A mathematical model of the rat kidney. III. Ammonia transport.Am J Physiol Renal Physiol. 2021 Jun 1;320(6):F1059-F1079. doi: 10.1152/ajprenal.00008.2021. Epub 2021 Mar 29. Am J Physiol Renal Physiol. 2021. PMID: 33779315 Free PMC article.
References
-
- Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. The Journal of Clinical Investigation. 1980. May;65(5):1162–1173. 10.1172/JCI109771 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

