Hormonal and intrauterine methods for contraception for women aged 25 years and younger
- PMID: 26280888
- PMCID: PMC9239531
- DOI: 10.1002/14651858.CD009805.pub3
Hormonal and intrauterine methods for contraception for women aged 25 years and younger
Abstract
Background: Women between the ages of 15 and 24 years have high rates of unintended pregnancy; over half of women in this age group want to avoid pregnancy. However, women under age 25 years have higher typical contraceptive failure rates within the first 12 months of use than older women. High discontinuation rates may also be a problem in this population. Concern that adolescents and young women will not find hormonal or intrauterine contraceptives acceptable or effective might deter healthcare providers from recommending these contraceptive methods.
Objectives: To compare the contraceptive failure (pregnancy) rates and to examine the continuation rates for hormonal and intrauterine contraception among young women aged 25 years and younger.
Search methods: We searched until 4 August 2015 for randomized controlled trials (RCTs) that compared hormonal or intrauterine methods of contraception in women aged 25 years and younger. Computerized databases included the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, POPLINE, CINAHL, and LILACS. We also searched for current trials via ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP).
Selection criteria: We considered RCTs in any language that reported the contraceptive failure rates for hormonal or intrauterine contraceptive methods, when compared with another contraceptive method, for women aged 25 years and younger. The other contraceptive method could have been another intrauterine contraceptive, another hormonal contraceptive or different dose of the same method, or a non-hormonal contraceptive. Treatment duration must have been at least three months. Eligible trials had to include the primary outcome of contraceptive failure rate (pregnancy). The secondary outcome was contraceptive continuation rate.
Data collection and analysis: One author conducted the primary data extraction and entered the information into Review Manager. Another author performed an independent data extraction and verified the initial entry. For dichotomous outcomes, we computed the Mantel-Haenszel odds ratio (OR) with 95% confidence interval (CI). Because of disparate interventions and outcome measures, we did not conduct meta-analysis.
Main results: Five trials met the inclusion criteria. The studies included a total of 1503 women, with a mean of 301 participants. The trials compared the following contraceptives: combined oral contraceptive (COC) versus transdermal contraceptive patch, vaginal contraceptive ring, or levonorgestrel intrauterine system 20 µg/day (LNG-IUS 20); LNG-IUS 12 µg/day (LNG-IUS 12) versus LNG-IUS 16 µg/day (LNG-IUS 16); and LNG-IUS 20 versus the copper T380A intrauterine device (IUD). In the trials comparing two different types of methods, the study arms did not differ significantly for contraceptive efficacy or continuation. The sample sizes were small for two of those studies. The only significant outcome was that a COC group had a higher proportion of women who discontinued for 'other personal reasons' compared with the group assigned to the LNG-IUS 20 (OR 0.27, 95% CI 0.09 to 0.85), which may have little clinic relevance. The trial comparing LNG-IUS 12 versus LNG-IUS 16 showed similar efficacy over one and three years. In three trials that examined different LNG-IUS, continuation was at least 75% at 6 to 36 months.
Authors' conclusions: We considered the overall quality of evidence to be moderate to low. Limitations were due to trial design or limited reporting. Different doses in the LNG-IUS did not appear to influence efficacy over three years. In another study, continuation of the LNG-IUS appeared at least as high as that for the COC. The current evidence was insufficient to compare efficacy and continuation rates for hormonal and intrauterine contraceptive methods in women aged 25 years and younger.
Conflict of interest statement
J Tang conducted the initial 2012 review and J Krashin conducted the 2015 update as fellows working under the mentorship of G Stuart, the principal investigator for Stuart 2005.
S Mody is a MERCK Nexplanon Trainer.
Figures
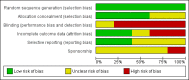








Update of
-
Hormonal and intrauterine methods for contraception for women aged 25 years and younger.Cochrane Database Syst Rev. 2012 Nov 14;11:CD009805. doi: 10.1002/14651858.CD009805.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Aug 17;(8):CD009805. doi: 10.1002/14651858.CD009805.pub3. PMID: 23152281 Updated.
References
References to studies included in this review
Godfrey 2010 {published data only}
-
- Godfrey EM, Memmel LM, Neustadt A, Shah M, Nicosia A, Moorthie M, et al. Intrauterine contraception for adolescents aged 14‐18 years: a multicenter randomized pilot study of levonorgestrel‐releasing intrauterine system compared to the Copper T 380A. Contraception 2010;81(2):123‐7. - PubMed
Kaunitz 2013 {published and unpublished data}
-
- Kaunitz AM, Dermout S, Tuppurainen M, Jensen J, Rosen K, Gemzell‐Danielsson K. Efficacy and safety of two low‐dose levonorgestrel intrauterine systems according to women's age: a global, multicentre, open‐label, randomized 3‐year Phase III Pearl Index study (conference poster abstract). European Journal of Contraception and Reproductive Health Care 2013;18(S1):S191‐2.
-
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low‐dose levonorgestrel intrauterine contraceptive systems. Obstetrics and Gynecology 2013;122(6):1205‐13. - PubMed
Stewart 2007 {published and unpublished data}
Stuart 2005 {published and unpublished data}
-
- Stuart GS, Moses BZ, Langston A, Heartwell SF. A pilot study in teenagers ‐ the oral contraceptive pill compared with the transdermal contraceptive patch (conference poster abstract). Obstetrics and Gynecology 2005;104(4 Suppl):93S.
Suhonen 2004 {published data only}
-
- Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrel‐releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception 2004;69(5):407‐12. - PubMed
References to studies excluded from this review
Berenson 2012 {published data only}
Borgatta 2014 {published data only}
-
- Borgatta L, Roth K, Rybowski S, Rosen K. A randomized phase III study comparing a new 13.5 mg levonorgestrel intrauterine contraceptive system with a combined oral contraceptive: analysis of efficacy and bleeding profiles. Fertility and Sterility 2014;102:e143.
-
- Buhling K, Rybowski S, Roth K, Rosen K. Randomised, multicentre, Phase III profiling study comparing a low‐dose levonorgestrel intrauterine system with combined oral contraception: analysis of bleeding, discontinuation rates and adverse events in the 18‐month comparative phase. European Journal of Contraception and Reproductive Health Care 2014;19(S1):S182.
Briggs 1983 {published data only}
-
- Briggs MH. A randomized prospective study of the metabolic effects of four low‐estrogen oral contraceptives. Journal of Reproductive Medicine 1983;28(1 Suppl):92‐100. - PubMed
Bryant 2015 {published data only}
-
- Bryant A. Etonogestrel‐releasing subdermal implant for adolescents in the postpartum period: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01666912 (accessed 4 December 2014).
Castaño 2012 {published data only}
-
- Castaño PM, Bynum JY, Andrés R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomized controlled trial. Obstetrics and Gynecology 2012;119(1):14‐20. - PubMed
-
- Hall KS, Castano P, Westhoff C. Menstrual distress symptoms, oral contraceptive attributed side effects and discontinuation among urban, multi‐ethnic adolescent and young adult women: is there a link?. Journal of Adolescent Health 2012;50(2):S20‐1.
Cobb 2007 {published data only}
-
- Cobb KL, Bachrach LK, Sowers M, Nieves J, Greendale GA, Kent KK, et al. The effect of oral contraceptives on bone mass and stress fractures in female runners. Medicine & Science in Sports & Exercise 2007;39(9):1464‐73. - PubMed
Creinin 2008 {published data only}
-
- Creinin MD, Meyn LA, Borgatta L, Barnhart K, Jensen J, Burke AE, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstetrics and Gynecology 2008;111(2 Pt 1):267‐77. - PubMed
Davis 2005 {published data only}
-
- Davis AR, Westhoff C, O'Connell K, Gallagher N. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstetrics and Gynecology 2005;106(1):97‐104. - PubMed
Gilliam 2010 {published data only}
-
- Gilliam ML, Neustadt A, Kozloski M, Mistretta S, Tilmon S, Godfrey E. Adherence and acceptability of the contraceptive ring compared with the pill among students: a randomized controlled trial. Obstetrics and Gynecology 2010;115(3):503‐10. - PubMed
Jensen 2012 {published data only}
-
- Jensen JT, Garie SG, Trummer D, Elliesen J. Bleeding profile of a flexible extended regimen of ethinylestradiol/drospirenone in US women: an open‐label, three‐arm, active‐controlled, multicenter study. Contraception 2012;86(2):110‐8. - PubMed
Kaunitz 2014 {published data only}
-
- Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR, Rubin A, et al. Low‐dose levonorgestrel and ethinyl estradiol patch and pill: a randomized controlled trial. Obstetrics and Gynecology 2014;123(2 Pt 1):295‐303. - PubMed
Larsson 1979 {published data only}
-
- Larsson B, Hagström B, Viberg L, Anker C, Hamberger L, Lindhe BA. Low risk of pelvic inflammatory disease in young never‐pregnant women using Gravigard. Contraception 1979;20(3):291‐5. - PubMed
Li 2011 {published data only}
-
- Li Y, Zhang SM, Chen F, Zhang CY, Li YP, Zhou J, et al. A multi‐center randomized controlled trial of intrauterine device use in Chinese women. Zhonghua Yi Xue Za Zhi 2011;91(45):3172‐5. - PubMed
Mansour 2011 {published data only}
-
- Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17beta‐oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. European Journal of Contraception and Reproductive Health Care 2011;16(6):430‐43. - PMC - PubMed
Meirik 2013 {published data only}
-
- Meirik O, Brache V, Orawan K, Habib N A, Schmidt J, Ortayli N, et al. A multicenter randomized clinical trial of one‐rod etonogestrel and two‐rod levonorgestrel contraceptive implants with nonrandomized copper‐IUD controls: methodology and insertion data. Contraception 2013;87(1):113‐20. - PubMed
Nanda 2014 {published data only}
-
- Nanda K, Lendvay A, Kwok C, Tolley E, Dube K, Brache V. Continuous compared with cyclic use of oral contraceptive pills in the Dominican Republic: a randomized controlled trial. Obstetrics and Gynecology 2014; Vol. 123, issue 5:1012‐22. - PubMed
Peterson 1991 {published data only}
-
- Petersen KR, Brooks L, Jacobsen N, Skoby SO. Clinical performance of intrauterine devices in nulligravidae: is the length of the endometrial cavity of significance?. Acta Europaea Fertilitatis 1991;22(4):225‐8. - PubMed
Rickert 2007 {published data only}
-
- Rickert VI, Tiezzi L, Lipshutz J, León J, Vaughan RD, Westhoff C. Depo Now: preventing unintended pregnancies among adolescents and young adults. Journal of Adolescent Health 2007;401(1):22‐8. - PubMed
Stephenson 2013 {published data only}
-
- Stephenson J, Shawe J, Panicker S, Brima N, Copas A, Sauer U, et al. Randomized trial of the effect of tailored versus standard use of the combined oral contraceptive pill on continuation rates at 1 year. Contraception 2013;88(4):523‐31. - PubMed
Strokosch 2006 {published data only}
-
- Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double‐blind, placebo‐controlled study. Journal of Adolescent Health 2006;39(6):819‐27. - PubMed
Tang 2012 {published data only}
-
- Tang YH, Feng ZJ. Comparative study of clinical effects of VCu IUD and TCu380A IUD were used on women who once been done cesarean section. Zhonghua Yi Xue Za Zhi 2012;92(12):1209‐11. - PubMed
Thomas 2005 {published data only}
-
- Thomas AG, Klihr‐Beall S, Siqueira L, Horing I, Zhang J. Concentration of depot medroxyprogesterone acetate and pain scores in adolescents: a randomized clinical trial. Contraception 2005;72(2):126‐9. - PubMed
Tuppurainen 2014 {published data only}
-
- Tuppurainen M, Lukkari‐lax E, Grunert J, Rybowski S. A 12‐month multicenter, randomized phase III study comparing a 13.5 mg levonorgestrel intrauterine contraceptive system with the etonogestrel subdermal contraceptive implant in women aged 18‐35 years. Fertility and Sterility 2014;102(3):e142. - PubMed
van der Straten 2010 {published data only}
-
- Straten A, Sahin‐Hodoglugil N, Clouse K, Mtetwa S, Chirenje MZ. Feasibility and potential acceptability of three cervical barriers among vulnerable young women in Zimbabwe. Journal of Family Planning & Reproductive Health Care 2010;36(1):13‐9. - PubMed
Wang 2013 {published data only}
-
- Wang LY, Li SZ, Wu SY, Zhao YH, Wang Y. A random control study of indomethacin‐containing MYCu intrauterine contraceptive device for 60 months. Zhonghua Yi Xue Za Zhi 2013;93(7):496‐9. - PubMed
Westhoff 2007 {published data only}
-
- Westhoff C, Heartwell S, Edwards S, Zieman M, Cushman L, Robilotto C, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstetrics and Gynecology 2007;109(6):1270‐6. - PubMed
Westhoff 2012 {published data only}
-
- Westhoff C, Kaunitz AM, Korver T, Sommer W, Bahamondes L, Darney P, et al. Efficacy, safety, and tolerability of a monophasic oral contraceptive containing nomegestrol acetate and 17beta‐estradiol: a randomized controlled trial. Obstetrics and Gynecology 2012;119(5):989‐99. - PubMed
Additional references
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Darroch 2011
-
- Darroch JE, Sedgh G, Ball H. Contraceptive Technologies: Responding to Women's Needs. New York: Guttmacher Institute, 2011.
DHHS 2008
-
- U.S. Department of Health and Human Services. Health and human services funding for abstinence education, education for teen pregnancy and HIV/STD prevention, and other programs that address adolescent sexual activity, 2008. aspe.hhs.gov/hsp/08/AbstinenceEducation (accessed 03 April 2011).
Finer 2014
Gavin 2009
-
- Gavin L, MacKay AP, Brown K, Harrier S, Ventura SJ, Kann L, et al. Sexual and reproductive health of persons aged 10‐24 years ‐ United States, 2002‐2007. Morbidity and Mortality Weekly Report. Surveillance Summaries 2009;58(6):1‐58. - PubMed
Grimes 2007
-
- Grimes DA, Lopez LM, Manion C, Schulz KF. Cochrane systematic reviews of IUD trials: lessons learned. Contraception 2007;75(6 Suppl):S55‐9. - PubMed
Guttmacher 2010
-
- Guttmacher Institute. Facts on satisfying the need for contraception in developing countries ‐ updated November 2010. New York: Guttmacher Institute, 2010.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Jones 2012
-
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006‐2010, and changes in patterns of use since 1995. National Health Statistics Reports 2012;18(60):1‐25. - PubMed
Kost 2008
MacQuarrie 2014
-
- MacQuarrie K. Unmet need for family planning among young women: levels and trends. Rockville (MD): ICF International DHS; 2014. Comparative Reports No. 34.
Mosher 2010
-
- Mosher WD, Jones J. Use of contraception in the United States: 1982‐2008. National Center for Health Statistics. Vital Health Statistics 2010;23(29):1‐44. - PubMed
Nelson 2013
-
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low‐dose levonorgestrel intrauterine contraceptive systems. Obstetrics and Gynecology 2013;122(6):1205‐13. - PubMed
O'Neil‐Callahan 2013
Patton 2009
-
- Patton GC, Coffey C, Sawyer SM, Viner RM, Haller DM, Bose K, et al. Global patterns of mortality in young people: a systematic analysis of population health data. Lancet 2009;374(9693):881‐92. - PubMed
Raine 2011
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenstock 2012
Schulz 2002
-
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359(9306):614‐8. - PubMed
Schulz 2010
Secura 2014
Shah 2012
-
- Shah IH, Ahman E. Unsafe abortion differentials in 2008 by age and developing country region: high burden among young women. Reproductive Health Matters 2012;20(39):169‐73. - PubMed
Strauss 2005
-
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005.
Trussell 2011
United Nations 2013
-
- United Nations. Millennium Development Goals and Beyond 2015. www.un.org/millenniumgoals/bkgd.shtml (accessed 6 April 2015).
UNPF 2010
-
- United Nations Population Fund. 2010 Update for the MDG Database: Adolescent Birth Rate. Geneva: United Nations Population Fund, 2010.
WHO 2011
-
- World Health Organization. Adolescent health. www.who.int/topics/adolescent_health/en/ (accessed 03 April 2011).
WHO 2014
-
- World Health Organization. Unsafe abortion: global and regional estimates of the incidence of unsafe abortion and associated mortality in 2008. Sixth edition, 2014. www.who.int/reproductivehealth/publications/unsafe_abortion/978924150111... (accessed 3 June 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

