The Future of Pharmaceutical Manufacturing Sciences
- PMID: 26280993
- PMCID: PMC4973848
- DOI: 10.1002/jps.24594
The Future of Pharmaceutical Manufacturing Sciences
Abstract
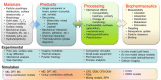
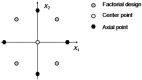
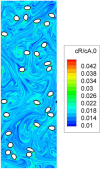
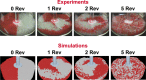
The entire pharmaceutical sector is in an urgent need of both innovative technological solutions and fundamental scientific work, enabling the production of highly engineered drug products. Commercial-scale manufacturing of complex drug delivery systems (DDSs) using the existing technologies is challenging. This review covers important elements of manufacturing sciences, beginning with risk management strategies and design of experiments (DoE) techniques. Experimental techniques should, where possible, be supported by computational approaches. With that regard, state-of-art mechanistic process modeling techniques are described in detail. Implementation of materials science tools paves the way to molecular-based processing of future DDSs. A snapshot of some of the existing tools is presented. Additionally, general engineering principles are discussed covering process measurement and process control solutions. Last part of the review addresses future manufacturing solutions, covering continuous processing and, specifically, hot-melt processing and printing-based technologies. Finally, challenges related to implementing these technologies as a part of future health care systems are discussed.
Keywords: in silico modeling; materials science; mathematical model; process analytical technology (PAT); quality by design (QBD).
© 2015 The Authors. Journal of Pharmaceutical Sciences published by Wiley Periodicals, Inc. and the American Pharmacists Association.
Figures










References
-
- Yu L. 2008. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm Res 25(4):781–791. - PubMed
-
- Rathore AS, Winkle H. 2009. Quality by design for biopharmaceuticals. Nat Biotechnol 27(1):26–34. - PubMed
-
- Oksanen CA, Muñoz S García. 2010. Process modeling and control in drug development and manufacturing. Comput Chem Eng 34(7):1007–1008.
-
- Reklaitis GV, Khinast J, Muzzio F. 2010. Pharmaceutical engineering science—New approaches to pharmaceutical development and manufacturing. Chem Eng Sci 65(21):iv–vii.
-
- Callis JB, Illman DL, Kowalski BR. 1987. Process analytical chemistry. Anal Chem 59(9):624A–637A.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

