Systemic modeling myeloma-osteoclast interactions under normoxic/hypoxic condition using a novel computational approach
- PMID: 26282073
- PMCID: PMC4539608
- DOI: 10.1038/srep13291
Systemic modeling myeloma-osteoclast interactions under normoxic/hypoxic condition using a novel computational approach
Abstract
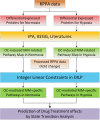
Interaction of myeloma cells with osteoclasts (OC) can enhance tumor cell expansion through activation of complex signaling transduction networks. Both cells reside in the bone marrow, a hypoxic niche. How OC-myeloma interaction in a hypoxic environment affects myeloma cell growth and their response to drug treatment is poorly understood. In this study, we i) cultured myeloma cells in the presence/absence of OCs under normoxia and hypoxia conditions and did protein profiling analysis using reverse phase protein array; ii) computationally developed an Integer Linear Programming approach to infer OC-mediated myeloma cell-specific signaling pathways under normoxic and hypoxic conditions. Our modeling analysis indicated that in the presence OCs, (1) cell growth-associated signaling pathways, PI3K/AKT and MEK/ERK, were activated and apoptotic regulatory proteins, BAX and BIM, down-regulated under normoxic condition; (2) β1 Integrin/FAK signaling pathway was activated in myeloma cells under hypoxic condition. Simulation of drug treatment effects by perturbing the inferred cell-specific pathways showed that targeting myeloma cells with the combination of PI3K and integrin inhibitors potentially (1) inhibited cell proliferation by reducing the expression/activation of NF-κB, S6, c-Myc, and c-Jun under normoxic condition; (2) blocked myeloma cell migration and invasion by reducing the expression of FAK and PKC under hypoxic condition.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

