Case-control study and case series of pseudohyperphosphatemia during exposure to liposomal amphotericin B
- PMID: 26282423
- PMCID: PMC4604351
- DOI: 10.1128/AAC.01306-15
Case-control study and case series of pseudohyperphosphatemia during exposure to liposomal amphotericin B
Abstract
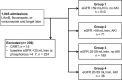
Pseudohyperphosphatemia due to an interaction between liposomal amphotericin B and the Beckman Coulter PHOSm assay occurs sporadically and remains underrecognized in clinical practice. This retrospective case-control study compares the incidences of hyperphosphatemia in adult inpatients exposed to liposomal amphotericin B or a triazole. A case series of patients with confirmed pseudohyperphosphatemia is described. A total of 80 exposures to liposomal amphotericin B and 726 exposures to triazoles were identified. Among subjects without chronic kidney disease and no concomitant acute kidney injury, hyperphosphatemia occurred more often during liposomal amphotericin B therapy than during triazole therapy (40% [14/35 cases] versus 10% [47/475 cases] of cases; P < 0.01; adjusted odds ratio, 5.2 [95% confidence interval {CI}, 2.3 to 11.9]). Among individuals with chronic kidney disease and no concomitant acute kidney injury, hyperphosphatemia also occurred more often during liposomal amphotericin B exposure (59% [10/17 cases] versus 20% [34/172 cases] of cases; P < 0.01; adjusted odds ratio, 6.0 [95% CI, 2.0 to 18.0]). When acute kidney injury occurred during antifungal exposure, the frequencies of hyperphosphatemia were not different between treatments. Seven episodes of unexpected hyperphosphatemia during liposomal amphotericin B exposure prompted a confirmatory test using an endpoint-based assay that found lower serum phosphorus levels (median difference of 2.5 mg/dl [range, 0.6 to 3.6 mg/dl]). Liposomal amphotericin B exposure confers a higher likelihood of developing hyperphosphatemia than that with exposure to a triazole antifungal, which is likely attributable to pseudohyperphosphatemia. Elevated phosphorus levels in patients receiving liposomal amphotericin B at institutions using the Beckman Coulter PHOSm assay should be interpreted cautiously.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Gilead Sciences, Inc. March 2012. AmBisome® package insert. Gilead Sciences, Inc., San Dimas, CA: http://www.gilead.com/pdf/ambisome_pi.pdf Accessed 3 August 2012.
-
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Dismukes WE, Holcenberg JS. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 340:764–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

