Clinical Course of Oxaliplatin-Induced Neuropathy: Results From the Randomized Phase III Trial N08CB (Alliance)
- PMID: 26282635
- PMCID: PMC4606060
- DOI: 10.1200/JCO.2014.58.8533
Clinical Course of Oxaliplatin-Induced Neuropathy: Results From the Randomized Phase III Trial N08CB (Alliance)
Abstract
Purpose: Given that the clinical course of oxaliplatin-induced neuropathy is not well defined, the current study was performed to better understand clinical parameters associated with its presentation.
Methods: Acute and chronic neuropathy was evaluated in patients receiving adjuvant FOLFOX (fluorouracil, leucovorin, and oxaliplatin) on study N08CB (North Central Cancer Treatment Group, Alliance). Acute neuropathy was assessed by having patients complete daily questionnaires for 6 days with each cycle of FOLFOX. Before each dose of FOLFOX and as long as 18 months after chemotherapy cessation, chronic neurotoxicity was assessed with use of the 20-item, European Organisation for Research and Treatment of Cancer quality-of-life questionnaire for patients with chemotherapy-induced peripheral neuropathy.
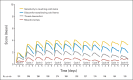
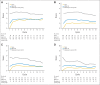
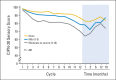
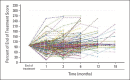
Results: Three hundred eight (89%) of the 346 patients had at least one symptom of acute neuropathy with the first cycle of FOLFOX; these symptoms included sensitivity to touching cold items (71%), sensitivity to swallowing cold items (71%), throat discomfort (63%), or muscle cramps (42%). Acute symptoms peaked at day 3 and improved, although they did not always resolve completely between treatments. These symptoms were about twice as severe in cycles 2 through 12 as they were in cycle 1. For chronic neurotoxicity, tingling was the most severe symptom, followed by numbness and then pain. During chemotherapy, symptoms in the hands were more prominent than they were in the feet; by 18 months, symptoms were more severe in the feet than they were in the hands. Patients with more severe acute neuropathy during the first cycle of therapy experienced more chronic sensory neurotoxicity (P < .0001).
Conclusion: Acute oxaliplatin-induced neuropathy symptoms do not always completely resolve between treatment cycles and are only half as severe on the first cycle as compared with subsequent cycles. There is a correlation between the severities of acute and chronic neuropathies.
Trial registration: ClinicalTrials.gov NCT00316914.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



References
-
- Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. - PubMed
-
- Pietrangeli A, Leandri M, Terzoli E, et al. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56:13–16. - PubMed
-
- Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438–444. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

