Evaluation of the Safety and Benefit of Phase I Oncology Trials for Patients With Primary CNS Tumors
- PMID: 26282642
- PMCID: PMC4857195
- DOI: 10.1200/JCO.2015.61.1525
Evaluation of the Safety and Benefit of Phase I Oncology Trials for Patients With Primary CNS Tumors
Abstract
Purpose: Patients with high-grade gliomas (HGG) are frequently excluded from first-in-human solid tumor trials because of perceived poor prognosis, excessive toxicities, concomitant drug interactions, and poor efficacy. We conducted an analysis of outcomes from select, single-agent phase I studies in patients with HGG. We compared outcomes to pooled analysis of published studies in solid tumors with various molecular and cytotoxic drugs evaluated as single agents or as combinations.
Patient and methods: Individual records of patients with recurrent HGG enrolled onto Adult Brain Tumor Consortium trials of single-agent, cytotoxic or molecular agents from 2000 to 2008 were analyzed for baseline characteristics, toxicities, responses, and survival.
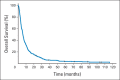
Results: Our analysis included 327 patients with advanced, refractory HGG who were enrolled onto eight trials involving targeted molecular (n=5) and cytotoxic (n=3) therapies. At enrollment, patients had a median Karnofsky performance score of 90 and median age of 52 years; 62% were men, 63% had glioblastoma, and the median number of prior systemic chemotherapies was one. Baseline laboratory values were in an acceptable range to meet eligibility criteria. Patients were on the study for a median of two cycles (range, <one to 56 cycles), and 96% were evaluable for primary end points. During cycle 1, grade≥3 nonhematologic and grade≥4 hematologic toxicities were 5% (28 of 565 adverse events) and 0.9% (five of 565 adverse events), respectively, and 66% of these occurred at the highest dose level. There was one death attributed to drug. Overall response rate (complete and partial response) was 5.5%. Median progression-free and overall survival times were 1.8 and 6 months, respectively.
Conclusion: Patients with HGG who meet standard eligibility criteria may be good candidates for solid tumor phase I studies with single-agent molecular or cytotoxic drugs with favorable preclinical rationale and pharmacokinetic properties in this population.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Similar articles
-
Efficacy of alectinib in central nervous system metastases in crizotinib-resistant ALK-positive non-small-cell lung cancer: Comparison of RECIST 1.1 and RANO-HGG criteria.Eur J Cancer. 2017 Sep;82:27-33. doi: 10.1016/j.ejca.2017.05.019. Epub 2017 Jul 10. Eur J Cancer. 2017. PMID: 28646771 Clinical Trial.
-
Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial.Lancet Oncol. 2015 Oct;16(13):1324-34. doi: 10.1016/S1470-2045(15)00188-6. Epub 2015 Sep 3. Lancet Oncol. 2015. PMID: 26342236 Clinical Trial.
-
Primary dissemination of high-grade gliomas in children: experiences from four studies of the Pediatric Oncology and Hematology Society of the German Language Group (GPOH).J Neurooncol. 2005 Apr;72(2):179-83. doi: 10.1007/s11060-004-3546-5. J Neurooncol. 2005. PMID: 15925999 Clinical Trial.
-
A Systematic Review of Pediatric Phase I Trials in Oncology: Toxicity and Outcomes in the Era of Targeted Therapies.Oncologist. 2020 Jun;25(6):532-540. doi: 10.1634/theoncologist.2019-0615. Epub 2020 Jan 14. Oncologist. 2020. PMID: 31943534 Free PMC article.
-
A comparison of safety and efficacy of cytotoxic versus molecularly targeted drugs in pediatric phase I solid tumor oncology trials.Pediatr Blood Cancer. 2017 Mar;64(3). doi: 10.1002/pbc.26258. Epub 2016 Sep 22. Pediatr Blood Cancer. 2017. PMID: 27654490
Cited by
-
Design and conduct of theranostic trials in neuro-oncology: Challenges and opportunities.Neuro Oncol. 2024 Dec 9;26(Supplement_9):S199-S207. doi: 10.1093/neuonc/noae162. Neuro Oncol. 2024. PMID: 39368109 Review.
-
Glioblastoma Vaccines as Promising Immune-Therapeutics: Challenges and Current Status.Vaccines (Basel). 2024 Jun 12;12(6):655. doi: 10.3390/vaccines12060655. Vaccines (Basel). 2024. PMID: 38932383 Free PMC article. Review.
-
Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions.Neuro Oncol. 2020 Aug 17;22(8):1073-1113. doi: 10.1093/neuonc/noaa106. Neuro Oncol. 2020. PMID: 32328653 Free PMC article. Review.
-
Profiles of brain metastases: Prioritization of therapeutic targets.Int J Cancer. 2018 Dec 1;143(11):3019-3026. doi: 10.1002/ijc.31624. Epub 2018 Oct 9. Int J Cancer. 2018. PMID: 29923182 Free PMC article.
-
Barriers to accrual and enrollment in brain tumor trials.Neuro Oncol. 2019 Sep 6;21(9):1100-1117. doi: 10.1093/neuonc/noz104. Neuro Oncol. 2019. PMID: 31175826 Free PMC article. Review.
References
-
- Eisenhauer EA, Twelves C, Buyse ME. Phase I Cancer Clinical Trials: A Practical Guide (ed 1) New York, NY: Oxford University Press; 2006.
-
- Carden CP, Sarker D, Postel-Vinay S, et al. Can molecular biomarker-based patient selection in phase I trials accelerate anticancer drug development? Drug Discov Today. 2010;15:88–97. - PubMed
-
- Gounder MM, Spriggs DR. Inclusion of patients with brain metastases in phase I trials: An unmet need. Clin Cancer Res. 2011;17:3855–3857. - PubMed