Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center
- PMID: 26282644
- PMCID: PMC5087335
- DOI: 10.1200/JCO.2015.60.8448
Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center
Abstract
Purpose: Ipilimumab is a standard treatment for metastatic melanoma, but immune-related adverse events (irAEs) are common and can be severe. We reviewed our large, contemporary experience with ipilimumab treatment outside of clinical trials to determine the frequency of use of systemic corticosteroid or anti-tumor necrosis factor α (anti-TNFα) therapy and the effect of these therapies on overall survival (OS) and time to treatment failure (TTF).
Patients and methods: We reviewed retrospectively the medical records of patients with melanoma who had received treatment between April 2011 and July 2013 with ipilimumab at the standard dose of 3 mg/kg. We collected data on patient demographics, previous and subsequent treatments, number of ipilimumab doses, irAEs and how they were treated, and overall survival.
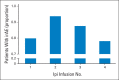
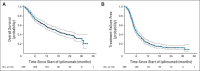
Results: Of the 298 patients, 254 (85%) experienced an irAE of any grade. Fifty-six patients (19%) discontinued therapy because of an irAE, most commonly diarrhea. Overall, 103 patients (35%) required systemic corticosteroid treatment for an irAE; 29 (10%) also required anti-TNFα therapy. Defining TTF as either starting a new treatment or death, estimated median TTF was 5.7 months. Twelve percent of patients experienced long-term disease control without receiving additional antimelanoma therapy. OS and TTF were not affected by the occurrence of irAEs or the need for systemic corticosteroids.
Conclusion: IrAEs are common in patients treated with ipilimumab. In our experience, approximately one-third of ipilimumab-treated patients required systemic corticosteroids, and almost one-third of those required further immune suppression with anti-TNFα therapy. Practitioners and patients should be prepared to treat irAEs and should understand that such treatment does not affect OS or TTF.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures

Comment in
-
Reply to A. Indini et al.J Clin Oncol. 2016 Mar 20;34(9):1018-9. doi: 10.1200/JCO.2015.65.7007. Epub 2016 Jan 19. J Clin Oncol. 2016. PMID: 26786917 No abstract available.
-
Immune Suppression and Response to Ipilimumab: Assessing Risk-to-Benefit Ratio.J Clin Oncol. 2016 Mar 20;34(9):1017-8. doi: 10.1200/JCO.2015.65.0028. Epub 2016 Jan 19. J Clin Oncol. 2016. PMID: 26786928 No abstract available.
References
-
- Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. - PubMed
-
- Princeton, NJ: Bristol-Myers Squibb Pharmaceuticals; 2012. Yervoy (ipilimumab) Immune-Mediated Adverse Reaction Management Guide.
-
- Johnston RL, Lutzky J, Chodhry A, et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci. 2009;54:2538–2540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

