The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison
- PMID: 26282839
- PMCID: PMC4989998
- DOI: 10.1016/j.jcf.2015.07.012
The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison
Abstract
Background: Circadian variation in renal toxicity of aminoglycosides has been demonstrated in animal and human studies. People with CF are frequently prescribed aminoglycosides. Altered pharmacokinetics of aminoglycosides are predictive of toxicity.
Aim: To investigate whether the time of day of aminoglycoside administration modulates renal excretion of tobramycin and toxicity in children with CF. To determine whether circadian rhythms are disrupted in children with CF during hospital admission.
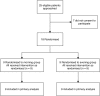
Methods: Children (age 5-18years) with CF scheduled for tobramycin therapy were randomly allocated to receive tobramycin at 0800 or 2000h. Serum tobramycin levels were drawn at 1h and between 3.5 and 5h post-infusion between days 5 and 9 of therapy. Melatonin levels were measured serially at intervals from 1800h in the evening until 1200h on the next day. Circadian rhythm was categorised as normal when dim light melatonin onset was demonstrated between 1800 and 2200h and/or peak melatonin levels were observed during the night. Weight and spirometry were measured at the start and end of the therapy. Urinary biomarkers of kidney toxicity (KIM1, NAG, NGAL, IL-18 and CysC) were assayed at the start and end of the course of tobramycin.
Results: Eighteen children were recruited to the study. There were no differences in renal clearance between the morning and evening groups. The increase in urinary KIM-1 was greater in the evening dosage group compared to the morning group (mean difference, 0.73ng/mg; 95% CI, 0.14 to 1.32; p=0.018). There were no differences in the other urinary biomarkers. There was normal circadian rhythm in 7/11 participants (64%).
Conclusions: Renal elimination of tobramycin was not affected by the time of day of administration. Urinary KIM-1 raises the possibility of greater nephrotoxicity with evening administration. Four children showed disturbed circadian rhythm and high melatonin levels (ClinicalTrials.gov NCT01207245).
Keywords: Aminoglycosides; Circadian rhythm; Cystic fibrosis; Toxicity.
Copyright © 2015. Published by Elsevier B.V.
Figures



References
-
- Trust U.K.C.F. CF Trust; 2014. UK Cystic Fibrosis Registry.
-
- Smyth A., Lewis S., Bertenshaw C., Choonara I., McGaw J., Watson A. Case–control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2008;63(6):532–535. [2008 June 1] - PubMed
-
- Al-Aloul M., Miller H., Alapati S., Stockton P., L M., Walshaw M. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39(1):15–20. - PubMed
-
- Touw D.J., Vinks A.A., Heijerman H.G., Bakker W. Validation of tobramycin monitoring in adolescent and adult patients with cystic fibrosis. Ther Drug Monit. Feb 1993;15(1):52–59. [PubMed PMID: 8451782. Epub 1993/02/01. eng.] - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

