Stoichiometry for α-bungarotoxin block of α7 acetylcholine receptors
- PMID: 26282895
- PMCID: PMC4544739
- DOI: 10.1038/ncomms9057
Stoichiometry for α-bungarotoxin block of α7 acetylcholine receptors
Abstract
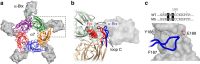
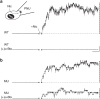
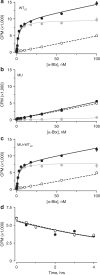
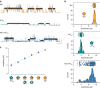
α-Bungarotoxin (α-Btx) binds to the five agonist binding sites on the homopentameric α7-acetylcholine receptor, yet the number of bound α-Btx molecules required to prevent agonist-induced channel opening remains unknown. To determine the stoichiometry for α-Btx blockade, we generate receptors comprised of wild-type and α-Btx-resistant subunits, tag one of the subunit types with conductance mutations to report subunit stoichiometry, and following incubation with α-Btx, monitor opening of individual receptor channels with defined subunit stoichiometry. We find that a single α-Btx-sensitive subunit confers nearly maximal suppression of channel opening, despite four binding sites remaining unoccupied by α-Btx and accessible to the agonist. Given structural evidence that α-Btx locks the agonist binding site in an inactive conformation, we conclude that the dominant mechanism of antagonism is non-competitive, originating from conformational arrest of the binding sites, and that the five α7 subunits are interdependent and maintain conformational symmetry in the open channel state.
Figures



References
-
- Chang C. C. & Lee C. Y. Isolation of neurotoxins from the venom of bungarus multicinctus and their modes of neuromuscular blocking action. Arch. Int. Pharmacodyn. Ther. 144, 241–257 (1963). - PubMed
-
- Betzel C. et al.. The refined crystal structure of α-cobratoxin from Naja naja siamensis at 2.4-Å resolution. J. Biol. Chem. 266, 21530–21536 (1991). - PubMed
-
- Neubig R. R. & Cohen J. B. Equilibrium binding of [3H]tubocurarine and [3H]acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry 18, 5464–5475 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

