Constitutive and ghrelin-dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons
- PMID: 26283199
- PMCID: PMC4555474
- DOI: 10.1085/jgp.201511383
Constitutive and ghrelin-dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons
Abstract
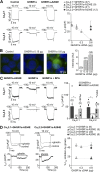
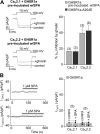
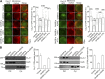
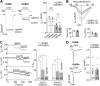
The growth hormone secretagogue receptor type 1a (GHSR1a) has the highest known constitutive activity of any G protein-coupled receptor (GPCR). GHSR1a mediates the action of the hormone ghrelin, and its activation increases transcriptional and electrical activity in hypothalamic neurons. Although GHSR1a is present at GABAergic presynaptic terminals, its effect on neurotransmitter release remains unclear. The activities of the voltage-gated calcium channels, CaV2.1 and CaV2.2, which mediate neurotransmitter release at presynaptic terminals, are modulated by many GPCRs. Here, we show that both constitutive and agonist-dependent GHSR1a activity elicit a strong impairment of CaV2.1 and CaV2.2 currents in rat and mouse hypothalamic neurons and in a heterologous expression system. Constitutive GHSR1a activity reduces CaV2 currents by a Gi/o-dependent mechanism that involves persistent reduction in channel density at the plasma membrane, whereas ghrelin-dependent GHSR1a inhibition is reversible and involves altered CaV2 gating via a Gq-dependent pathway. Thus, GHSR1a differentially inhibits CaV2 channels by Gi/o or Gq protein pathways depending on its mode of activation. Moreover, we present evidence suggesting that GHSR1a-mediated inhibition of CaV2 attenuates GABA release in hypothalamic neurons, a mechanism that could contribute to neuronal activation through the disinhibition of postsynaptic neurons.
© 2015 López Soto et al.
Figures


Comment in
-
All roads lead to presynaptic calcium channel inhibition by the ghrelin receptor: Separate agonist-dependent and -independent signaling pathways.J Gen Physiol. 2015 Sep;146(3):201-4. doi: 10.1085/jgp.201511462. Epub 2015 Aug 17. J Gen Physiol. 2015. PMID: 26283201 Free PMC article. No abstract available.
References
-
- Andrews Z.B., Erion D., Beiler R., Liu Z.W., Abizaid A., Zigman J., Elsworth J.D., Savitt J.M., DiMarchi R., Tschoep M., et al. . 2009. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J. Neurosci. 29:14057–14065. 10.1523/JNEUROSCI.3890-09.2009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

