Enteral glutamine supplementation in critically ill patients: a systematic review and meta-analysis
- PMID: 26283217
- PMCID: PMC4539709
- DOI: 10.1186/s13054-015-1002-x
Enteral glutamine supplementation in critically ill patients: a systematic review and meta-analysis
Abstract
Introduction: Glutamine (GLN) has been suggested to have a beneficial influence on outcomes of critically ill patients. However, recent large-scale trials have suggested harm associated with GLN supplementation. Recently, systematic reviews on the use of parenteral GLN have been published; however, less information is available on the role of enteral GLN. Therefore, the aim of this systematic review was to study the effects of enteral GLN supplementation in patients with critical illness.
Methods: We identified randomized controlled trials conducted from 1980 to 2014 with enterally administered GLN in adult critically ill patients. Studies of parenteral GLN only or combined enteral-parenteral GLN were excluded. The methodological quality of studies was scored, and trial data were statistically combined. We examined a priori the treatment effects in subgroups of trials of burn and trauma patients.
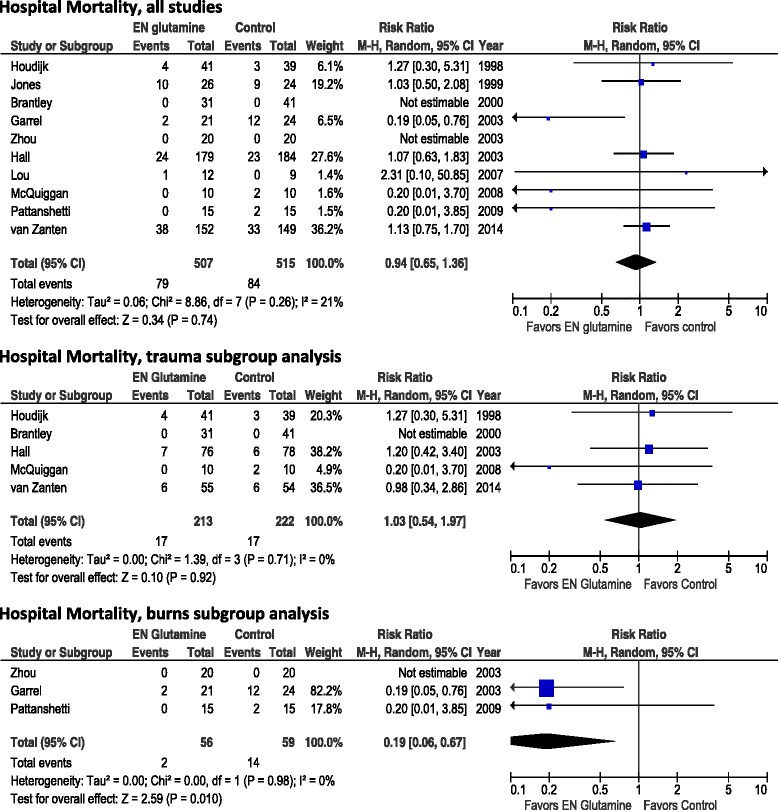
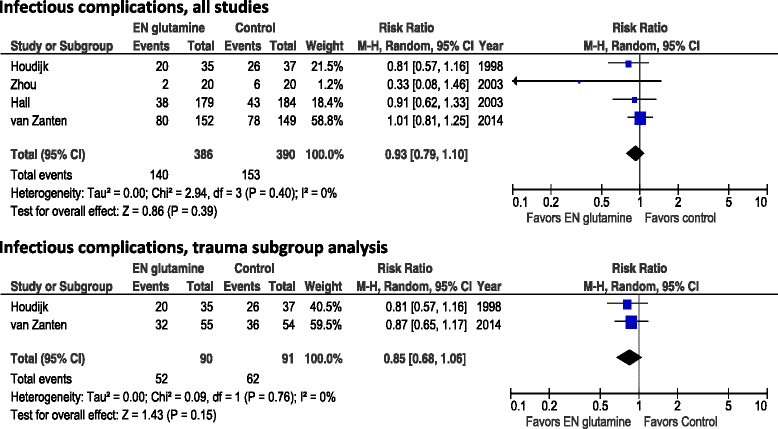
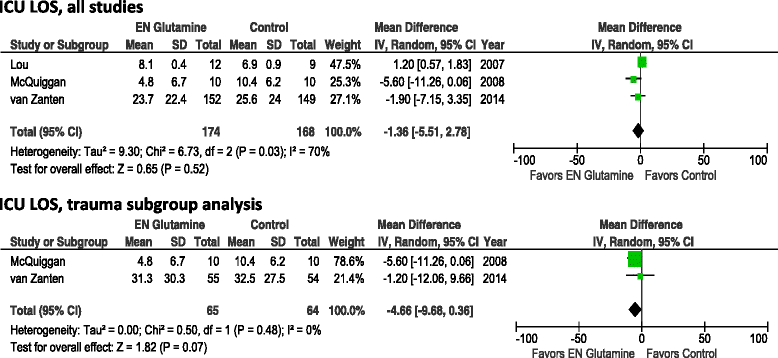
Results: A total of 11 studies involving 1079 adult critically ill patients and enteral GLN supplementation were identified. Enteral GLN supplementation was not associated with a reduction of hospital mortality (risk ratio [RR] 0.94, 95% confidence interval [CI] 0.65-1.36; p = 0.74), infectious complications (RR 0.93, 95% CI 0.79-1.10; p = 0.39) or stay in the intensive care unit (weighted mean difference [WMD] -1.36 days, 95% CI -5.51 to 2.78; p = 0.52). However, there was a significant reduction in hospital stay (WMD 4.73 days, 95% CI -8.53 to -0.90; p = 0.02). In the subset of studies of patients with burns, enteral GLN supplementation was associated with significant reductions in hospital mortality (RR 0.19, 95% 0.06-0.67; p = 0.010) and hospital stay (WMD -9.16, 95% CI -15.06 to -3.26; p = 0.002). There was no effect in trauma patients.
Conclusions: Enteral GLN supplementation does not confer significant clinical benefit in critically ill patients, with the exception of reduced hospital stay. There may be a significant benefit in patients with burns, but data are sparse and larger randomized trials are warranted to confirm this effect.
Figures

Comment in
-
Enteral glutamine supplementation in critically ill patients.Crit Care. 2015 Nov 24;19:405. doi: 10.1186/s13054-015-1111-6. Crit Care. 2015. PMID: 26597862 Free PMC article. No abstract available.
References
-
- Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

