Viral fusion protein transmembrane domain adopts β-strand structure to facilitate membrane topological changes for virus-cell fusion
- PMID: 26283363
- PMCID: PMC4568205
- DOI: 10.1073/pnas.1501430112
Viral fusion protein transmembrane domain adopts β-strand structure to facilitate membrane topological changes for virus-cell fusion
Abstract
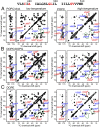
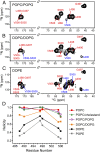
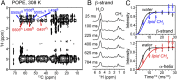
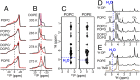
The C-terminal transmembrane domain (TMD) of viral fusion proteins such as HIV gp41 and influenza hemagglutinin (HA) is traditionally viewed as a passive α-helical anchor of the protein to the virus envelope during its merger with the cell membrane. The conformation, dynamics, and lipid interaction of these fusion protein TMDs have so far eluded high-resolution structure characterization because of their highly hydrophobic nature. Using magic-angle-spinning solid-state NMR spectroscopy, we show that the TMD of the parainfluenza virus 5 (PIV5) fusion protein adopts lipid-dependent conformations and interactions with the membrane and water. In phosphatidylcholine (PC) and phosphatidylglycerol (PG) membranes, the TMD is predominantly α-helical, but in phosphatidylethanolamine (PE) membranes, the TMD changes significantly to the β-strand conformation. Measured order parameters indicate that the strand segments are immobilized and thus oligomerized. (31)P NMR spectra and small-angle X-ray scattering (SAXS) data show that this β-strand-rich conformation converts the PE membrane to a bicontinuous cubic phase, which is rich in negative Gaussian curvature that is characteristic of hemifusion intermediates and fusion pores. (1)H-(31)P 2D correlation spectra and (2)H spectra show that the PE membrane with or without the TMD is much less hydrated than PC and PG membranes, suggesting that the TMD works with the natural dehydration tendency of PE to facilitate membrane merger. These results suggest a new viral-fusion model in which the TMD actively promotes membrane topological changes during fusion using the β-strand as the fusogenic conformation.
Keywords: conformational polymorphism; membrane curvature; peptide–membrane interactions; small-angle X-ray scattering; solid-state NMR spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

