Multiple component networks support working memory in prefrontal cortex
- PMID: 26283366
- PMCID: PMC4568266
- DOI: 10.1073/pnas.1504172112
Multiple component networks support working memory in prefrontal cortex
Erratum in
-
Correction to Supporting Information for Markowitz et al., Multiple component networks support working memory in prefrontal cortex.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):E5555. doi: 10.1073/pnas.1517476112. Epub 2015 Sep 14. Proc Natl Acad Sci U S A. 2015. PMID: 26371320 Free PMC article. No abstract available.
Abstract
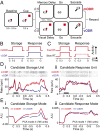
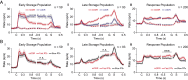
Lateral prefrontal cortex (PFC) is regarded as the hub of the brain's working memory (WM) system, but it remains unclear whether WM is supported by a single distributed network or multiple specialized network components in this region. To investigate this problem, we recorded from neurons in PFC while monkeys made delayed eye movements guided by memory or vision. We show that neuronal responses during these tasks map to three anatomically specific modes of persistent activity. The first two modes encode early and late forms of information storage, whereas the third mode encodes response preparation. Neurons that reflect these modes are concentrated at different anatomical locations in PFC and exhibit distinct patterns of coordinated firing rates and spike timing during WM, consistent with distinct networks. These findings support multiple component models of WM and consequently predict distinct failures that could contribute to neurologic dysfunction.
Keywords: coherence; macaque; prefrontal cortex; working memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Baddeley AD. Working Memory. Clarendon; Oxford: 1986.
-
- Miyake A, Shah P. In: Models of Working Memory. Miyake A, Shah P, editors. Cambridge University Press; New York: 1999.
-
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. - PubMed
-
- Cohen JD, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. - PubMed
-
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386(6625):608–611. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

