Talin determines the nanoscale architecture of focal adhesions
- PMID: 26283369
- PMCID: PMC4568271
- DOI: 10.1073/pnas.1512025112
Talin determines the nanoscale architecture of focal adhesions
Abstract
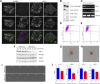
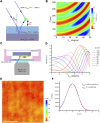
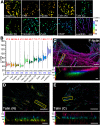
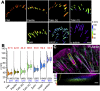
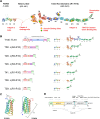
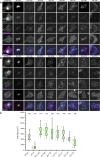
Insight into how molecular machines perform their biological functions depends on knowledge of the spatial organization of the components, their connectivity, geometry, and organizational hierarchy. However, these parameters are difficult to determine in multicomponent assemblies such as integrin-based focal adhesions (FAs). We have previously applied 3D superresolution fluorescence microscopy to probe the spatial organization of major FA components, observing a nanoscale stratification of proteins between integrins and the actin cytoskeleton. Here we combine superresolution imaging techniques with a protein engineering approach to investigate how such nanoscale architecture arises. We demonstrate that talin plays a key structural role in regulating the nanoscale architecture of FAs, akin to a molecular ruler. Talin diagonally spans the FA core, with its N terminus at the membrane and C terminus demarcating the FA/stress fiber interface. In contrast, vinculin is found to be dispensable for specification of FA nanoscale architecture. Recombinant analogs of talin with modified lengths recapitulated its polarized orientation but altered the FA/stress fiber interface in a linear manner, consistent with its modular structure, and implicating the integrin-talin-actin complex as the primary mechanical linkage in FAs. Talin was found to be ∼97 nm in length and oriented at ∼15° relative to the plasma membrane. Our results identify talin as the primary determinant of FA nanoscale organization and suggest how multiple cellular forces may be integrated at adhesion sites.
Keywords: focal adhesions; mechanobiology; nanoscale architecture; superresolution microscopy; talin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2(11):793–805. - PubMed
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. - PubMed
-
- Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–1709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

