Dual allosteric activation mechanisms in monomeric human glucokinase
- PMID: 26283387
- PMCID: PMC4577146
- DOI: 10.1073/pnas.1506664112
Dual allosteric activation mechanisms in monomeric human glucokinase
Abstract
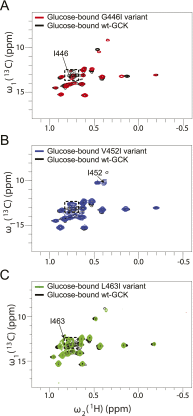
Cooperativity in human glucokinase (GCK), the body's primary glucose sensor and a major determinant of glucose homeostatic diseases, is fundamentally different from textbook models of allostery because GCK is monomeric and contains only one glucose-binding site. Prior work has demonstrated that millisecond timescale order-disorder transitions within the enzyme's small domain govern cooperativity. Here, using limited proteolysis, we map the site of disorder in unliganded GCK to a 30-residue active-site loop that closes upon glucose binding. Positional randomization of the loop, coupled with genetic selection in a glucokinase-deficient bacterium, uncovers a hyperactive GCK variant with substantially reduced cooperativity. Biochemical and structural analysis of this loop variant and GCK variants associated with hyperinsulinemic hypoglycemia reveal two distinct mechanisms of enzyme activation. In α-type activation, glucose affinity is increased, the proteolytic susceptibility of the active site loop is suppressed and the (1)H-(13)C heteronuclear multiple quantum coherence (HMQC) spectrum of (13)C-Ile-labeled enzyme resembles the glucose-bound state. In β-type activation, glucose affinity is largely unchanged, proteolytic susceptibility of the loop is enhanced, and the (1)H-(13)C HMQC spectrum reveals no perturbation in ensemble structure. Leveraging both activation mechanisms, we engineer a fully noncooperative GCK variant, whose functional properties are indistinguishable from other hexokinase isozymes, and which displays a 100-fold increase in catalytic efficiency over wild-type GCK. This work elucidates specific structural features responsible for generating allostery in a monomeric enzyme and suggests a general strategy for engineering cooperativity into proteins that lack the structural framework typical of traditional allosteric systems.
Keywords: allostery; diabetes; glucokinase; intrinsic disorder; monomeric cooperativity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Comment in
-
Allostery vs. "allokairy".Proc Natl Acad Sci U S A. 2015 Sep 15;112(37):11430-1. doi: 10.1073/pnas.1515239112. Epub 2015 Sep 8. Proc Natl Acad Sci U S A. 2015. PMID: 26372953 Free PMC article. No abstract available.
References
-
- Changeux JP. 50 years of allosteric interactions: The twists and turns of the models. Nat Rev Mol Cell Biol. 2013;14(12):819–829. - PubMed
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Koshland DE, Jr, Némethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5(1):365–385. - PubMed
-
- Macol CP, Tsuruta H, Stec B, Kantrowitz ER. Direct structural evidence for a concerted allosteric transition in Escherichia coli aspartate transcarbamoylase. Nat Struct Biol. 2001;8(5):423–426. - PubMed
-
- Freiburger L, et al. Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme. Nat Chem Biol. 2014;10(11):937–942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

