Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study
- PMID: 26283655
- PMCID: PMC4590523
- DOI: 10.1161/CIRCULATIONAHA.115.016871
Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study
Abstract
Background: There is a lack of data on the comparative efficacy and procedural safety of open irrigated radiofrequency (RF) and cryoballoon catheter (CB) ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation.
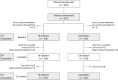
Methods and results: In a prospective, noninferiority study, 315 patients were randomly assigned to RF (n=159) or CB (n=156) ablation. The primary end point was freedom from atrial arrhythmia with absence of persistent complications. Patients were largely comparable between groups with more vascular disease in the RF group (8.2% versus 2.6% for CB; P=0.028). The primary end point at 12 months was achieved by 70.7% with RF and 73.6% with CB (multiple procedure success), including 31 redo procedures in each group (19.5% of RF versus 19.9% of CB; P=0.933). For the intention-to-treat population, noninferiority of CB was revealed for the predefined inferiority margin (risk difference, 0.029; 95% confidence interval, -0.074 to 0.132; P<0.001). Rates at 6 months were 63.1% and 64.1% for the RF and CB groups (single procedure success), and noninferiority was confirmed (risk difference, 0.010; 95% confidence interval, -0.097 to 0.116; P=0.002). Periprocedural complications for the index procedure were more frequent in the CB group (5.0% RF, 12.2% CB; P=0.022) with a significant difference in phrenic nerve palsies (0% RF, 5.8% CB; P=0.002).
Conclusion: This large, prospective, randomized, controlled study demonstrates noninferiority of CB ablation versus RF ablation for treating patients with paroxysmal atrial fibrillation.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00774566.
Trial registration: ClinicalTrials.gov NCT01490814.
Keywords: arrhythmias, cardiac; atrial fibrillation; catheter ablation.
© 2015 The Authors.
Figures
References
-
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.CIR.98.10.946. - PubMed
-
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/S0002-9343(02)01236-6S. - PubMed
-
- Van Gelder IC, Crijns HJ, Tieleman RG, Brügemann J, De Kam PJ, Gosselink AT, Verheugt FW, Lie KI. Chronic atrial fibrillation: success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med. 1996;156:2585–2592. doi: 10.1001/archinte.1996.00440210109011. - PubMed
-
- Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012;5:CD005049. doi: 10.1002/14651858.CD005049.pub3. - PubMed
-
- Hollands JM, Gowan M, Riney JN, Deal EN, Kates AM. Role of new drugs for management of atrial fibrillation. Ann Pharmacother. 2012;46:1656–1670. doi: 10.1345/aph.1R155. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

