Interactions between Ibrutinib and Anti-CD20 Antibodies: Competing Effects on the Outcome of Combination Therapy
- PMID: 26283682
- PMCID: PMC4703510
- DOI: 10.1158/1078-0432.CCR-15-1304
Interactions between Ibrutinib and Anti-CD20 Antibodies: Competing Effects on the Outcome of Combination Therapy
Abstract
Purpose: Clinical trials of ibrutinib combined with anti-CD20 monoclonal antibodies (mAb) for chronic lymphocytic leukemia (CLL) report encouraging results. Paradoxically, in preclinical studies, in vitro ibrutinib was reported to decrease CD20 expression and inhibit cellular effector mechanisms. We therefore set out to investigate effects of in vivo ibrutinib treatment that could explain this paradox.
Experimental design: Patients received single-agent ibrutinib (420 mg daily) on an investigator-initiated phase II trial. Serial blood samples were collected pretreatment and during treatment for ex vivo functional assays to examine the effects on CLL cell susceptibility to anti-CD20 mAbs.
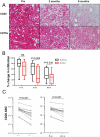
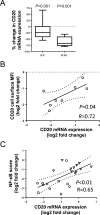
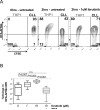
Results: We demonstrate that CD20 expression on ibrutinib was rapidly and persistently downregulated (median reduction 74%, day 28, P < 0.001) compared with baseline. Concomitantly, CD20 mRNA was decreased concurrent with reduced NF-κB signaling. An NF-κB binding site in the promoter of MS4A1 (encoding CD20) and downregulation of CD20 by NF-κB inhibitors support a direct transcriptional effect. Ex vivo, tumor cells from patients on ibrutinib were less susceptible to anti-CD20 mAb-mediated complement-dependent cytotoxicity than pretreatment cells (median reduction 75%, P < 0.001); however, opsonization by the complement protein C3d, which targets cells for phagocytosis, was relatively maintained. Expression of decay-accelerating factor (CD55) decreased on ibrutinib, providing a likely mechanism for the preserved C3d opsonization. In addition, ibrutinib significantly inhibited trogocytosis, a major contributor to antigen loss and tumor escape during mAb therapy.
Conclusions: Our data indicate that ibrutinib promotes both positive and negative interactions with anti-CD20 mAbs, suggesting that successfully harnessing maximal antitumor effects of such combinations requires further investigation.
©2015 American Association for Cancer Research.
Figures



References
-
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:88–94. - PMC - PubMed
-
- Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in Previously Treated Waldenström’s Macroglobulinemia. New England Journal of Medicine. 2015;372:1430–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

