Glutamate transporter homolog-based model predicts that anion-π interaction is the mechanism for the voltage-dependent response of prestin
- PMID: 26283790
- PMCID: PMC4591817
- DOI: 10.1074/jbc.M115.649962
Glutamate transporter homolog-based model predicts that anion-π interaction is the mechanism for the voltage-dependent response of prestin
Abstract
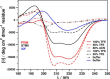
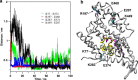
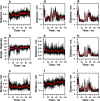
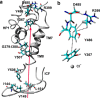
Prestin is the motor protein of cochlear outer hair cells. Its unique capability to perform direct, rapid, and reciprocal electromechanical conversion depends on membrane potential and interaction with intracellular anions. How prestin senses the voltage change and interacts with anions are still unknown. Our three-dimensional model of prestin using molecular dynamics simulations predicts that prestin contains eight transmembrane-spanning segments and two helical re-entry loops and that tyrosyl residues are the structural specialization of the molecule for the unique function of prestin. Using site-directed mutagenesis and electrophysiological techniques, we confirmed that residues Tyr(367), Tyr(486), Tyr(501), and Tyr(508) contribute to anion binding, interacting with intracellular anions through novel anion-π interactions. Such weak interactions, sensitive to voltage and mechanical stimulation, confer prestin with a unique capability to perform electromechanical and mechanoelectric conversions with exquisite sensitivity. This novel mechanism is completely different from all known mechanisms seen in ion channels, transporters, and motor proteins.
Keywords: anion-π interactions; electrophysiology; hair cell; homology modeling; molecular dynamics; prestin; protein structure; transmembrane domain.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Liberman M. C., Gao J., He D. Z., Wu X., Jia S., Zuo J. (2002) Prestin is required for outer hair cell motility and the cochlear amplifier. Nature 419, 300–304 - PubMed
-
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196 - PubMed
-
- Zheng J., Shen W., He D. Z., Long K. B., Madison L. D., Dallos P. (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

