Variable phenotypic expressivity in inbred retinal degeneration mouse lines: A comparative study of C3H/HeOu and FVB/N rd1 mice
- PMID: 26283863
- PMCID: PMC4522243
Variable phenotypic expressivity in inbred retinal degeneration mouse lines: A comparative study of C3H/HeOu and FVB/N rd1 mice
Abstract
Purpose: Recent advances in optogenetics and gene therapy have led to promising new treatment strategies for blindness caused by retinal photoreceptor loss. Preclinical studies often rely on the retinal degeneration 1 (rd1 or Pde6b(rd1)) retinitis pigmentosa (RP) mouse model. The rd1 founder mutation is present in more than 100 actively used mouse lines. Since secondary genetic traits are well-known to modify the phenotypic progression of photoreceptor degeneration in animal models and human patients with RP, negligence of the genetic background in the rd1 mouse model is unwarranted. Moreover, the success of various potential therapies, including optogenetic gene therapy and prosthetic implants, depends on the progress of retinal degeneration, which might differ between rd1 mice. To examine the prospect of phenotypic expressivity in the rd1 mouse model, we compared the progress of retinal degeneration in two common rd1 lines, C3H/HeOu and FVB/N.
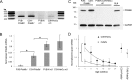
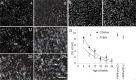
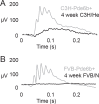
Methods: We followed retinal degeneration over 24 weeks in FVB/N, C3H/HeOu, and congenic Pde6b(+) seeing mouse lines, using a range of experimental techniques including extracellular recordings from retinal ganglion cells, PCR quantification of cone opsin and Pde6b transcripts, in vivo flash electroretinogram (ERG), and behavioral optokinetic reflex (OKR) recordings.
Results: We demonstrated a substantial difference in the speed of retinal degeneration and accompanying loss of visual function between the two rd1 lines. Photoreceptor degeneration and loss of vision were faster with an earlier onset in the FVB/N mice compared to C3H/HeOu mice, whereas the performance of the Pde6b(+) mice did not differ significantly in any of the tests. By postnatal week 4, the FVB/N mice expressed significantly less cone opsin and Pde6b mRNA and had neither ERG nor OKR responses. At 12 weeks of age, the retinal ganglion cells of the FVB/N mice had lost all light responses. In contrast, 4-week-old C3H/HeOu mice still had ERG and OKR responses, and we still recorded light responses from C3H/HeOu retinal ganglion cells until the age of 24 weeks. These results show that genetic background plays an important role in the rd1 mouse pathology.
Conclusions: Analogous to human RP, the mouse genetic background strongly influences the rd1 phenotype. Thus, different rd1 mouse lines may follow different timelines of retinal degeneration, making exact knowledge of genetic background imperative in all studies that use rd1 models.
Figures




References
-
- Busskamp V, Roska B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr Opin Neurobiol. 2011;21:942–6. - PubMed
-
- Cronin T, Vandenberghe L, Hantz P, Juttner J, Reimann A, Kacsó A, Huckfeldt R, Busskamp V, Kohler H, Lagali P, Roska B, Bennett J. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med. 2014;6:1175–90. - PMC - PubMed
-
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

