Sphingosine kinase 2-deficiency mediated changes in spinal pain processing
- PMID: 26283908
- PMCID: PMC4522551
- DOI: 10.3389/fnmol.2015.00029
Sphingosine kinase 2-deficiency mediated changes in spinal pain processing
Abstract
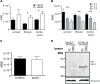
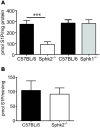
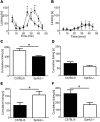
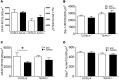
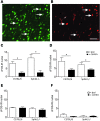
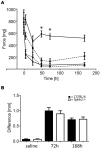
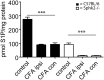
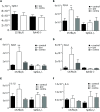
Chronic pain is one of the most burdensome health issues facing the planet (as costly as diabetes and cancer combined), and in desperate need for new diagnostic targets leading to better therapies. The bioactive lipid sphingosine 1-phosphate (S1P) and its receptors have recently been shown to modulate nociceptive signaling at the level of peripheral nociceptors and central neurons. However, the exact role of S1P generating enzymes, in particular sphingosine kinase 2 (Sphk2), in nociception remains unknown. We found that both sphingosine kinases, Sphk1 and Sphk2, were expressed in spinal cord (SC) with higher levels of Sphk2 mRNA compared to Sphk1. All three Sphk2 mRNA-isoforms were present with the Sphk2.1 mRNA showing the highest relative expression. Mice deficient in Sphk2 (Sphk2(-/-)) showed in contrast to mice deficient in Sphk1 (Sphk1(-/-)) substantially lower spinal S1P levels compared to wild-type C57BL/6 mice. In the formalin model of acute peripheral inflammatory pain, Sphk2(-/-) mice showed facilitation of nociceptive transmission during the late response, whereas responses to early acute pain, and the number of c-Fos immunoreactive dorsal horn neurons were not different between Sphk2(-/-) and wild-type mice. Chronic peripheral inflammation (CPI) caused a bilateral increase in mechanical sensitivity in Sphk2(-/-) mice. Additionally, CPI increased the relative mRNA expression of P2X4 receptor, brain-derived neurotrophic factor and inducible nitric oxide synthase in the ipsilateral SC of wild-type but not Sphk2(-/-) mice. Similarly, Sphk2(-/-) mice showed in contrast to wild-type no CPI-dependent increase in areas of the dorsal horn immunoreactive for the microglia marker Iba-1 and the astrocyte marker Glial fibrillary acidic protein (GFAP). Our results suggest that the tightly regulated cell signaling enzyme Sphk2 may be a key component for facilitation of nociceptive circuits in the CNS leading to central sensitization and pain memory formation.
Keywords: CFA; RT-PCR; dorsal horn; formalin; knock-out mouse; sphingosine 1-phosphate.
Figures
Similar articles
-
The Functional Role of Sphingosine Kinase 2.Front Mol Biosci. 2021 May 14;8:683767. doi: 10.3389/fmolb.2021.683767. eCollection 2021. Front Mol Biosci. 2021. PMID: 34055895 Free PMC article. Review.
-
Sphingosine 1-phosphate modulates spinal nociceptive processing.J Biol Chem. 2008 Nov 21;283(47):32442-51. doi: 10.1074/jbc.M806410200. Epub 2008 Sep 19. J Biol Chem. 2008. PMID: 18805787
-
The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen.Mol Immunol. 2011 May;48(9-10):1139-48. doi: 10.1016/j.molimm.2011.02.007. Epub 2011 Mar 23. Mol Immunol. 2011. PMID: 21435724
-
Different Roles of Sphingosine Kinase 1 and 2 in Pancreatic Cancer Progression.J Surg Res. 2018 Dec;232:186-194. doi: 10.1016/j.jss.2018.06.019. Epub 2018 Jul 6. J Surg Res. 2018. PMID: 30463717
-
Regulation and function of sphingosine kinase 2 in diseases.Histol Histopathol. 2018 May;33(5):433-445. doi: 10.14670/HH-11-939. Epub 2017 Oct 19. Histol Histopathol. 2018. PMID: 29057430 Review.
Cited by
-
The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer.Mediators Inflamm. 2017;2017:4806541. doi: 10.1155/2017/4806541. Epub 2017 Nov 15. Mediators Inflamm. 2017. PMID: 29269995 Free PMC article. Review.
-
The Functional Role of Sphingosine Kinase 2.Front Mol Biosci. 2021 May 14;8:683767. doi: 10.3389/fmolb.2021.683767. eCollection 2021. Front Mol Biosci. 2021. PMID: 34055895 Free PMC article. Review.
-
miR-19a mediates the mechanism by which SPHK2 regulates hypopharyngeal squamous cell carcinoma progression through the PI3K/AKT axis.Am J Cancer Res. 2023 Jun 15;13(6):2342-2359. eCollection 2023. Am J Cancer Res. 2023. PMID: 37424828 Free PMC article.
-
Targeting the Sphingosine-1-Phosphate Axis for Developing Non-narcotic Pain Therapeutics.Trends Pharmacol Sci. 2020 Nov;41(11):851-867. doi: 10.1016/j.tips.2020.09.006. Epub 2020 Oct 1. Trends Pharmacol Sci. 2020. PMID: 33010954 Free PMC article. Review.
-
Cellular stress promotes NOD1/2-dependent inflammation via the endogenous metabolite sphingosine-1-phosphate.EMBO J. 2021 Jul 1;40(13):e106272. doi: 10.15252/embj.2020106272. Epub 2021 May 4. EMBO J. 2021. PMID: 33942347 Free PMC article.
References
-
- Beroukas D., Pitson S. M., Matusica D., Gibbins I. L., Kress M., Haberberger R. V. (2015). Sphingosine kinase 1 in murine dorsal root ganglia. AIMS Mol. Sci. 1, 22–33. 10.3934/molsci.2015.1.22 - DOI
-
- Camprubí-Robles M., Mair N., Andratsch M., Benetti C., Beroukas D., Rukwied R., et al. . (2013). Sphingosine-1-phosphate-induced nociceptor excitation and ongoing pain behavior in mice and humans is largely mediated by S1P3 receptor. J. Neurosci. 33, 2582–2592. 10.1523/JNEUROSCI.4479-12.2013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

