Myelin damage and repair in pathologic CNS: challenges and prospects
- PMID: 26283909
- PMCID: PMC4515562
- DOI: 10.3389/fnmol.2015.00035
Myelin damage and repair in pathologic CNS: challenges and prospects
Abstract
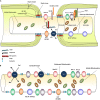
Injury to the central nervous system (CNS) results in oligodendrocyte cell death and progressive demyelination. Demyelinated axons undergo considerable physiological changes and molecular reorganizations that collectively result in axonal dysfunction, degeneration and loss of sensory and motor functions. Endogenous adult oligodendrocyte precursor cells and neural stem/progenitor cells contribute to the replacement of oligodendrocytes, however, the extent and quality of endogenous remyelination is suboptimal. Emerging evidence indicates that optimal remyelination is restricted by multiple factors including (i) low levels of factors that promote oligodendrogenesis; (ii) cell death among newly generated oligodendrocytes, (iii) inhibitory factors in the post-injury milieu that impede remyelination, and (iv) deficient expression of key growth factors essential for proper re-construction of a highly organized myelin sheath. Considering these challenges, over the past several years, a number of cell-based strategies have been developed to optimize remyelination therapeutically. Outcomes of these basic and preclinical discoveries are promising and signify the importance of remyelination as a mechanism for improving functions in CNS injuries. In this review, we provide an overview on: (1) the precise organization of myelinated axons and the reciprocal axo-myelin interactions that warrant properly balanced physiological activities within the CNS; (2) underlying cause of demyelination and the structural and functional consequences of demyelination in axons following injury and disease; (3) the endogenous mechanisms of oligodendrocyte replacement; (4) the modulatory role of reactive astrocytes and inflammatory cells in remyelination; and (5) the current status of cell-based therapies for promoting remyelination. Careful elucidation of the cellular and molecular mechanisms of demyelination in the pathologic CNS is a key to better understanding the impact of remyelination for CNS repair.
Keywords: astrocytes; cell therapy; demyelination; neural stem cells; oligodendrocyte precursor cells; oligodendrocytes; remyelination; spinal cord injury.
Figures

References
-
- Akiyama Y., Lankford K., Radtke C., Greer C. A., Kocsis J. D. (2004). Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene. Neuron Glia Biol. 1 47–55. 10.1017/S1740925X04000079 - DOI - PMC - PubMed
-
- All A. H., Bazley F. A., Gupta S., Pashai N., Hu C., Pourmorteza A., et al. (2012). Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS ONE 7:e47645 10.1371/journal.pone.0047645 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

