Evaluation of the amyloid beta-GFP fusion protein as a model of amyloid beta peptides-mediated aggregation: a study of DNAJB6 chaperone
- PMID: 26283911
- PMCID: PMC4515555
- DOI: 10.3389/fnmol.2015.00040
Evaluation of the amyloid beta-GFP fusion protein as a model of amyloid beta peptides-mediated aggregation: a study of DNAJB6 chaperone
Abstract
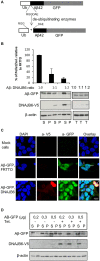
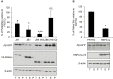
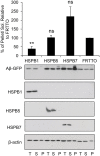
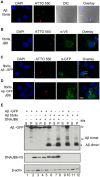
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by the accumulation and aggregation of extracellular amyloid β (Aβ) peptides and intracellular aggregation of hyper-phosphorylated tau protein. Recent evidence indicates that accumulation and aggregation of intracellular amyloid β peptides may also play a role in disease pathogenesis. This would suggest that intracellular Heat Shock Proteins (HSP) that maintain cellular protein homeostasis might be candidates for disease amelioration. We recently found that DNAJB6, a member of DNAJ family of heat shock proteins, effectively prevented the aggregation of short aggregation-prone peptides containing large poly glutamines (associated with CAG repeat diseases) both in vitro and in cells. Moreover, recent in vitro data showed that DNAJB6 can delay the aggregation of Aβ42 peptides. In this study, we investigated the ability of DNAJB6 to prevent the aggregation of extracellular and intracellular Aβ peptides using transfection of human embryonic kidney 293 (HEK293) cells with Aβ-green fluorescent protein (GFP) fusion construct and performing western blotting and immunofluorescence techniques. We found that DNAJB6 indeed suppresses Aβ-GFP aggregation, but not seeded aggregation initiated by extracellular Aβ peptides. Unexpectedly and unlike what we found for peptide-mediated aggregation, DNAJB6 required interaction with HSP70 to prevent the aggregation of the Aβ-GFP fusion protein and its J-domain was crucial for its anti-aggregation effect. In addition, other DNAJ proteins as well as HSPA1a overexpression also suppressed Aβ-GFP aggregation efficiently. Our findings suggest that Aβ aggregation differs from poly glutamine (Poly Q) peptide induced aggregation in terms of chaperone handling and sheds doubt on the usage of Aβ-GFP fusion construct for studying Aβ peptide aggregation in cells.
Keywords: Alzheimer’s disease; Aβ-GFP; DNAJB6; amyloid beta aggregation; chaperones; heat shock proteins.
Figures
Similar articles
-
Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation.J Biol Chem. 2014 Nov 7;289(45):31066-76. doi: 10.1074/jbc.M114.595124. Epub 2014 Sep 12. J Biol Chem. 2014. PMID: 25217638 Free PMC article.
-
Amyloid-β oligomers are captured by the DNAJB6 chaperone: Direct detection of interactions that can prevent primary nucleation.J Biol Chem. 2020 Jun 12;295(24):8135-8144. doi: 10.1074/jbc.RA120.013459. Epub 2020 Apr 29. J Biol Chem. 2020. PMID: 32350108 Free PMC article.
-
Conserved S/T Residues of the Human Chaperone DNAJB6 Are Required for Effective Inhibition of Aβ42 Amyloid Fibril Formation.Biochemistry. 2018 Aug 14;57(32):4891-4902. doi: 10.1021/acs.biochem.8b00353. Epub 2018 Jul 30. Biochemistry. 2018. PMID: 30024736
-
The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases.Front Neurosci. 2017 May 16;11:254. doi: 10.3389/fnins.2017.00254. eCollection 2017. Front Neurosci. 2017. PMID: 28559789 Free PMC article. Review.
-
Updates on Aβ Processing by Hsp90, BRICHOS, and Newly Reported Distinctive Chaperones.Biomolecules. 2023 Dec 22;14(1):16. doi: 10.3390/biom14010016. Biomolecules. 2023. PMID: 38254616 Free PMC article. Review.
Cited by
-
The roles of HSP40/DNAJ protein family in neurodegenerative diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Nov 25;51(5):640-646. doi: 10.3724/zdxbyxb-2021-0406. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36581576 Free PMC article. Review. English.
-
A Genetic Code Expansion-Derived Molecular Beacon for the Detection of Intracellular Amyloid-β Peptide Generation.Angew Chem Int Ed Engl. 2021 Feb 19;60(8):3934-3939. doi: 10.1002/anie.202010703. Epub 2020 Dec 15. Angew Chem Int Ed Engl. 2021. PMID: 33063327 Free PMC article.
-
Integrating single-nucleus sequence profiling to reveal the transcriptional dynamics of Alzheimer's disease, Parkinson's disease, and multiple sclerosis.J Transl Med. 2023 Sep 21;21(1):649. doi: 10.1186/s12967-023-04516-6. J Transl Med. 2023. PMID: 37735671 Free PMC article.
-
A Genetic Code Expansion-Derived Molecular Beacon for the Detection of Intracellular Amyloid-β Peptide Generation.Angew Chem Weinheim Bergstr Ger. 2021 Feb 19;133(8):3980-3985. doi: 10.1002/ange.202010703. Epub 2020 Dec 15. Angew Chem Weinheim Bergstr Ger. 2021. PMID: 38504667 Free PMC article.
-
The Molecular Chaperone DNAJB6, but Not DNAJB1, Suppresses the Seeded Aggregation of Alpha-Synuclein in Cells.Int J Mol Sci. 2019 Sep 11;20(18):4495. doi: 10.3390/ijms20184495. Int J Mol Sci. 2019. PMID: 31514384 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

