Elusive roles for reactive astrocytes in neurodegenerative diseases
- PMID: 26283915
- PMCID: PMC4522610
- DOI: 10.3389/fncel.2015.00278
Elusive roles for reactive astrocytes in neurodegenerative diseases
Abstract
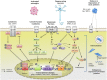
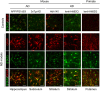
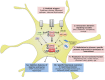
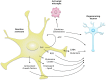
Astrocytes play crucial roles in the brain and are involved in the neuroinflammatory response. They become reactive in response to virtually all pathological situations in the brain such as axotomy, ischemia, infection, and neurodegenerative diseases (ND). Astrocyte reactivity was originally characterized by morphological changes (hypertrophy, remodeling of processes) and the overexpression of the intermediate filament glial fibrillary acidic protein (GFAP). However, it is unclear how the normal supportive functions of astrocytes are altered by their reactive state. In ND, in which neuronal dysfunction and astrocyte reactivity take place over several years or decades, the issue is even more complex and highly debated, with several conflicting reports published recently. In this review, we discuss studies addressing the contribution of reactive astrocytes to ND. We describe the molecular triggers leading to astrocyte reactivity during ND, examine how some key astrocyte functions may be enhanced or altered during the disease process, and discuss how astrocyte reactivity may globally affect ND progression. Finally we will consider the anticipated developments in this important field. With this review, we aim to show that the detailed study of reactive astrocytes may open new perspectives for ND.
Keywords: Alzheimer's disease; Huntington's disease; Parkinson's disease; amyotrophic lateral sclerosis; astrocyte reactivity; neuroinflammation; neuron-astrocyte interactions.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

