Hunting for origins of migraine pain: cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers
- PMID: 26283923
- PMCID: PMC4516892
- DOI: 10.3389/fncel.2015.00287
Hunting for origins of migraine pain: cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers
Abstract
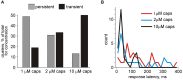
Trigeminal nerves in meninges are implicated in generation of nociceptive firing underlying migraine pain. However, the neurochemical mechanisms of nociceptive firing in meningeal trigeminal nerves are little understood. In this study, using suction electrode recordings from peripheral branches of the trigeminal nerve in isolated rat meninges, we analyzed spontaneous and capsaicin-induced orthodromic spiking activity. In control, biphasic single spikes with variable amplitude and shapes were observed. Application of the transient receptor potential vanilloid 1 (TRPV1) agonist capsaicin to meninges dramatically increased firing whereas the amplitudes and shapes of spikes remained essentially unchanged. This effect was antagonized by the specific TRPV1 antagonist capsazepine. Using the clustering approach, several groups of uniform spikes (clusters) were identified. The clustering approach combined with capsaicin application allowed us to detect and to distinguish "responder" (65%) from "non-responder" clusters (35%). Notably, responders fired spikes at frequencies exceeding 10 Hz, high enough to provide postsynaptic temporal summation of excitation at brainstem and spinal cord level. Almost all spikes were suppressed by tetrodotoxin (TTX) suggesting an involvement of the TTX-sensitive sodium channels in nociceptive signaling at the peripheral branches of trigeminal neurons. Our analysis also identified transient (desensitizing) and long-lasting (slowly desensitizing) responses to the continuous application of capsaicin. Thus, the persistent activation of nociceptors in capsaicin-sensitive nerve fibers shown here may be involved in trigeminal pain signaling and plasticity along with the release of migraine-related neuropeptides from TRPV1 positive neurons. Furthermore, cluster analysis could be widely used to characterize the temporal and neurochemical profiles of other pain transducers likely implicated in migraine.
Keywords: capsaicin; cluster analysis; pain; spike; trigeminal nerve.
Figures




Similar articles
-
Antidromic Spike Propagation and Dissimilar Expression of P2X, 5-HT, and TRPV1 Channels in Peripheral vs. Central Sensory Axons in Meninges.Front Cell Neurosci. 2021 Jan 15;14:623134. doi: 10.3389/fncel.2020.623134. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33519387 Free PMC article.
-
Differential inhibitory effects of endocannabinoids on neuronal firing of mouse meningeal afferents.J Headache Pain. 2025 May 12;26(1):112. doi: 10.1186/s10194-025-02041-z. J Headache Pain. 2025. PMID: 40355840 Free PMC article.
-
Mechanosensitive meningeal nociception via Piezo channels: Implications for pulsatile pain in migraine?Neuropharmacology. 2019 May 1;149:113-123. doi: 10.1016/j.neuropharm.2019.02.015. Epub 2019 Feb 13. Neuropharmacology. 2019. PMID: 30768945
-
Serotonergic mechanisms of trigeminal meningeal nociception: Implications for migraine pain.Neuropharmacology. 2017 Apr;116:160-173. doi: 10.1016/j.neuropharm.2016.12.024. Epub 2016 Dec 23. Neuropharmacology. 2017. PMID: 28025094
-
TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches.Int J Mol Sci. 2020 Jan 4;21(1):342. doi: 10.3390/ijms21010342. Int J Mol Sci. 2020. PMID: 31948011 Free PMC article. Review.
Cited by
-
Protective Effects of Hydrogen Sulfide Against the ATP-Induced Meningeal Nociception.Front Cell Neurosci. 2020 Sep 2;14:266. doi: 10.3389/fncel.2020.00266. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32982692 Free PMC article.
-
Purinergic Profiling of Regulatory T-cells in Patients With Episodic Migraine.Front Cell Neurosci. 2018 Sep 25;12:326. doi: 10.3389/fncel.2018.00326. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30319363 Free PMC article.
-
Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents.Front Cell Neurosci. 2017 Jul 27;11:226. doi: 10.3389/fncel.2017.00226. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28798669 Free PMC article.
-
Deciphering in silico the Role of Mutated Na V 1.1 Sodium Channels in Enhancing Trigeminal Nociception in Familial Hemiplegic Migraine Type 3.Front Cell Neurosci. 2021 May 31;15:644047. doi: 10.3389/fncel.2021.644047. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34135733 Free PMC article.
-
Activation of P2X7 Receptors in Peritoneal and Meningeal Mast Cells Detected by Uptake of Organic Dyes: Possible Purinergic Triggers of Neuroinflammation in Meninges.Front Cell Neurosci. 2019 Feb 13;13:45. doi: 10.3389/fncel.2019.00045. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30814932 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

