eIF2α Confers Cellular Tolerance to S. aureus α-Toxin
- PMID: 26284068
- PMCID: PMC4515601
- DOI: 10.3389/fimmu.2015.00383
eIF2α Confers Cellular Tolerance to S. aureus α-Toxin
Abstract
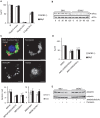
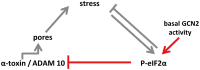
We report on the role of conserved stress-response pathways for cellular tolerance to a pore forming toxin. First, we observed that small molecular weight inhibitors including of eIF2α-phosphatase, jun-N-terminal kinase (JNK), and PI3-kinase sensitized normal mouse embryonal fibroblasts (MEFs) to the small pore forming S. aureus α-toxin. Sensitization depended on expression of mADAM10, the murine ortholog of a proposed high-affinity receptor for α-toxin in human cells. Similarly, eIF2α (S51A/S51A) MEFs, which harbor an Ala knock-in mutation at the regulated Ser51 phosphorylation site of eukaryotic translation initiation factor 2α, were hyper-sensitive to α-toxin. Inhibition of translation with cycloheximide did not mimic the tolerogenic effect of eIF2α-phosphorylation. Notably, eIF2α-dependent tolerance of MEFs was toxin-selective, as wild-type MEFs and eIF2α (S51A/S51A) MEFs exhibited virtually equal sensitivity to Vibrio cholerae cytolysin. Binding of S. aureus α-toxin to eIF2α (S51A/S51A) MEFs and toxicity in these cells were enhanced as compared to wild-type cells. This led to the unexpected finding that the mutant cells carried more ADAM10. Because basal phosphorylation of eIF2α in MEFs required amino acid deprivation-activated eIF2α-kinase 4/GCN2, the data reveal that basal activity of this kinase mediates tolerance of MEFs to α-toxin. Further, they suggest that modulation of ADAM10 is involved. During infection, bacterial growth may cause nutrient shortage in tissues, which might activate this response. Tolerance to α-toxin was robust in macrophages and did not depend on GCN2. However, JNKs appeared to play a role, suggesting differential cell type and toxin selectivity of tolerogenic stress responses. Understanding their function or failure will be important to comprehend anti-bacterial immune responses.
Keywords: EIF2AK4; MAPK; S. aureus α-toxin; cellular tolerance; pore forming toxins.
Figures
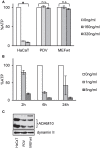
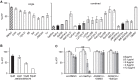

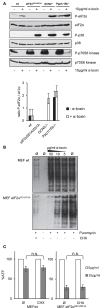
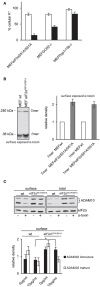
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

