How tree roots respond to drought
- PMID: 26284083
- PMCID: PMC4518277
- DOI: 10.3389/fpls.2015.00547
How tree roots respond to drought
Abstract
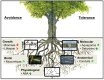
The ongoing climate change is characterized by increased temperatures and altered precipitation patterns. In addition, there has been an increase in both the frequency and intensity of extreme climatic events such as drought. Episodes of drought induce a series of interconnected effects, all of which have the potential to alter the carbon balance of forest ecosystems profoundly at different scales of plant organization and ecosystem functioning. During recent years, considerable progress has been made in the understanding of how aboveground parts of trees respond to drought and how these responses affect carbon assimilation. In contrast, processes of belowground parts are relatively underrepresented in research on climate change. In this review, we describe current knowledge about responses of tree roots to drought. Tree roots are capable of responding to drought through a variety of strategies that enable them to avoid and tolerate stress. Responses include root biomass adjustments, anatomical alterations, and physiological acclimations. The molecular mechanisms underlying these responses are characterized to some extent, and involve stress signaling and the induction of numerous genes, leading to the activation of tolerance pathways. In addition, mycorrhizas seem to play important protective roles. The current knowledge compiled in this review supports the view that tree roots are well equipped to withstand drought situations and maintain morphological and physiological functions as long as possible. Further, the reviewed literature demonstrates the important role of tree roots in the functioning of forest ecosystems and highlights the need for more research in this emerging field.
Keywords: abscisic acid; avoidance; carbon sequestration; hydraulic signals; molecular responses; mycorrhizas; tolerance; tree root traits.
Figures


References
-
- Allen C. D., Macalady A. K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259 660–684. 10.1016/j.foreco.2009.09.001 - DOI
-
- Almeida-Rodriguez A. M., Cooke J. E. K., Yeh F., Zwiazek J. J. (2010). Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii x balsamifera clones with different drought resistance strategies. Physiol. Plant. 140 321–333. 10.1111/j.1399-3054.2010.01405.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

