Plastid genomics in horticultural species: importance and applications for plant population genetics, evolution, and biotechnology
- PMID: 26284102
- PMCID: PMC4520007
- DOI: 10.3389/fpls.2015.00586
Plastid genomics in horticultural species: importance and applications for plant population genetics, evolution, and biotechnology
Abstract
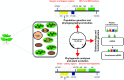
During the evolution of the eukaryotic cell, plastids, and mitochondria arose from an endosymbiotic process, which determined the presence of three genetic compartments into the incipient plant cell. After that, these three genetic materials from host and symbiont suffered several rearrangements, bringing on a complex interaction between nuclear and organellar gene products. Nowadays, plastids harbor a small genome with ∼130 genes in a 100-220 kb sequence in higher plants. Plastid genes are mostly highly conserved between plant species, being useful for phylogenetic analysis in higher taxa. However, intergenic spacers have a relatively higher mutation rate and are important markers to phylogeographical and plant population genetics analyses. The predominant uniparental inheritance of plastids is like a highly desirable feature for phylogeny studies. Moreover, the gene content and genome rearrangements are efficient tools to capture and understand evolutionary events between different plant species. Currently, genetic engineering of the plastid genome (plastome) offers a number of attractive advantages as high-level of foreign protein expression, marker gene excision, gene expression in operon and transgene containment because of maternal inheritance of plastid genome in most crops. Therefore, plastid genome can be used for adding new characteristics related to synthesis of metabolic compounds, biopharmaceutical, and tolerance to biotic and abiotic stresses. Here, we describe the importance and applications of plastid genome as tools for genetic and evolutionary studies, and plastid transformation focusing on increasing the performance of horticultural species in the field.
Keywords: conservation; horticultural crops; photosynthesis; plastid genetic engineering; plastome.
Figures
References
-
- Angioi S. A., Rau D., Rodriguez M., Logozzo G., Desiderio F., Papa R., et al. (2009b). Nuclear and chloroplast microsatellite diversity in Phaseolus vulgaris L. from Sardinia (Italy). Mol. Breeding 23 413–429. 10.1007/s11032-008-9245-8 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

