Medical interventions for high-grade vulval intraepithelial neoplasia
- PMID: 26284429
- PMCID: PMC6457779
- DOI: 10.1002/14651858.CD007924.pub3
Medical interventions for high-grade vulval intraepithelial neoplasia
Abstract
Background: This is an updated version of a review first published in theCochrane Database of Systematic Reviews, Issue 4, in 2011. Vulval intraepithelial neoplasia (VIN) is a pre-cancerous condition of the vulval skin and its incidence is increasing in women under 50 years. High-grade VIN (also called usual-type VIN (uVIN) or VIN 2/3 or high-grade vulval intraepithelial lesion) is associated with human papilloma virus (HPV) infection and may progress to vulval cancer, therefore is usually actively managed. There is no consensus on the optimal management of high-grade VIN; and the high morbidity and relapse rates associated with surgical interventions make less invasive interventions highly desirable.
Objectives: To evaluate the effectiveness and safety of medical (non-surgical) interventions for high-grade VIN.
Search methods: We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 3), MEDLINE and EMBASE (up to 30 March 2015). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria: Randomised controlled trials (RCTs) that assessed non-surgical interventions in women diagnosed with high-grade VIN.
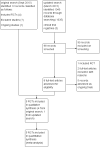
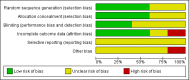
Data collection and analysis: We used Cochrane methodology with two review authors independently abstracting data and assessing risk of bias. Where possible, we synthesised data in meta-analyses using random effects methods.
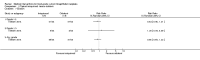
Main results: Five trials involving 297 women with high-grade VIN (defined by trial investigators as VIN 2/3 or VIN 3 or 'high-grade' lesions) met our inclusion criteria: three trials assessed the effectiveness of topical imiquimod versus placebo; one assessed topical cidofovir versus topical imiquimod; and one assessed low- versus high-dose indole-3-carbinol in similar types of participants. Three trials were at a moderate to low risk of bias, two were at a potentially high risk of bias.Meta-analysis of the three trials comparing topical imiquimod 5% cream to placebo found that women in the active treatment group were more likely to show an overall response (complete and partial response) to treatment at five to six months compared with the placebo group (Risk Ratio (RR) 11.95, 95% confidence interval (CI) 3.21 to 44.51; participants = 104; studies = 3; I(2) = 0%; high-quality evidence). A complete response at five to six months occurred in 36/62 (58%) and 0/42 (0%) participants in the active and placebo groups, respectively (RR 14.40, 95% CI 2.97 to 69.80; participants = 104; studies = 3; I(2) = 0%). A single trial reported 12-month follow-up, which revealed a sustained effect in overall response in favour of the active treatment arm at 12 months (RR 9.10, 95% CI 2.38 to 34.77; moderate-quality evidence), with 9/24 (38%) and 0/23 (0%) complete responses recorded in the active and placebo groups respectively. Progression to vulval cancer was also documented in this trial (one versus two participants in the active and placebo groups, respectively) and we assessed this evidence as low-quality. Only one trial reported adverse events, including erythema, erosion, pain and pruritis at the site of the lesion, which were more common in the imiquimod group. Dose reductions occurred more frequently in the active treatment group compared with the placebo group (19/47 versus 1/36 participants; RR 7.77, 95% CI 1.61 to 37.36; participants = 83; studies = 2; I(2) = 0%; high-quality evidence). Only one trial reported quality of life (QoL) and there were no significant differences between the imiquimod and placebo groups.For the imiquimod versus cidofovir trial, 180 women contributed data. The overall response at six months was similar for the imiquimod and cidofovir treatment groups with 52/91 (57%) versus 55/89 (62%) participants responding, respectively (RR 0.92, 95% CI 0.73 to 1.18). A complete response occurred in 41 women in each group (45% and 46%, respectively; RR 1.00, 95% CI 0.73 to 1.37). Although not statistically different, total adverse events were slightly more common in the imiquimod group of this trial with slightly more discontinuations occurring in this group. Longer term response data from this trial are expected.The small trial comparing two doses of indole-3-carbinol contributed limited data. We identified five ongoing randomised trials of various interventions for VIN.
Authors' conclusions: Topical imiquimod appears to be a safe and effective treatment for high-grade VIN (uVIN), even though local side-effects may necessitate dose reductions. However, longer term follow-up data are needed to corroborate the limited evidence that response to treatment is sustained, and to assess any effect on progression to vulval cancer. Available evidence suggests that topical cidofovir may be a good alternative to imiquimod; however, more evidence is needed, particularly regarding the relative effectiveness on longer term response and progression. We await the longer-term response data and the results of the five ongoing trials.
Conflict of interest statement
Andy Nordin is a co‐investigator in the Tristram 2014 trial.
Figures















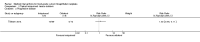
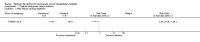






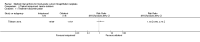
Update of
-
Medical interventions for high grade vulval intraepithelial neoplasia.Cochrane Database Syst Rev. 2011 Apr 13;(4):CD007924. doi: 10.1002/14651858.CD007924.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Aug 18;(8):CD007924. doi: 10.1002/14651858.CD007924.pub3. PMID: 21491403 Free PMC article. Updated.
References
References to studies included in this review
Mathiesen 2007 {published data only}
-
- Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double‐blinded study. Gynecologic Oncology 2007;107(2):219‐22. - PubMed
Naik 2006 {published data only}
-
- Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole‐3‐carbinol in the treatment of vulvar intraepithelial neoplasia. International Journal of Gynecological Cancer 2006; Vol. 16, issue 2:786‐90. - PubMed
Sterling 2005 {published data only}
-
- Sterling J C, Smith NA, Loo WJ, Cohen C, Neill S, Nicholson A, et al. Randomized, double blind, placebo‐controlled trial for treatment of high grade vulval intraepithelial neoplasia with imiquimod. Abstract FC06.1. Journal of the European Academy of Dermatology and Venereology 2005; Vol. 19, issue Suppl 2:22.
Tristram 2014 {published data only}
-
- Tristram A, Hurt CN, Madden T, Powell N, Man S, Hibbitts S, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT(3)VIN): a multicentre, open‐label, randomised, phase 2 trial. Lancet Oncology 2014;15(12):1361‐8. - PubMed
Van Seters 2008 {published data only}
-
- Terlou A, Seters M, Ewing PC, Aaronson NK, Gundy CM, Heijmans‐Antonissen C, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: Seven years median follow‐up of a randomized clinical trial. Gynecologic Oncology 2011;121(1):157‐62. - PubMed
-
- Seters M, Beurden M, Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. New England Journal of Medicine 2008;358(14):1465‐73. - PubMed
References to studies excluded from this review
Frega 2013 {published data only}
-
- Frega A, Sesti F, Sopracordevole F, Biamonti A, Scirpa P, Milazzo GN, et al. Imiquimod 5% cream versus cold knife excision for treatment of VIN 2/3: a five‐year follow‐up. Eur Rev Med Pharmacol Sci 2013;17(7):936‐40. - PubMed
Iavazzo 2008 {published data only}
-
- Iavazzo C, Pitsouni E, Athanasiou S, Falagas ME. Imiquimod for treatment of vulvar and vaginal intraepithelial neoplasia. International Journal of Gynaecology and Obstetrics 2008;101(1):3‐10. - PubMed
Mahto 2010 {published data only}
-
- Mahto M, Nathan M, O’Mahony C. More than a decade on: review of the use of imiquimod in lower anogenital intraepithelial neoplasia. International Journal of STD & AIDS 2010;21(1):8‐16. - PubMed
Mathiesen 2008 {published data only}
-
- Mathiesen O. Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double blinded study ‐ an answer. Gynecological Oncology 2008;109(3):431. - PubMed
Melamed 1965 {published data only}
-
- Melamed E L. Precancerous lesions of the vulva and therapeutic methods. Data from the Leningrad Municipal oncological dispensary. [Russian]. Voprosy 1965;Onkologii:85‐9. - PubMed
Spirtos 1990 {published data only}
-
- Spirtos N M, Smith L H, Teng N N. Prospective randomized trial of topical alpha‐interferon (alpha‐interferon gels) for the treatment of vulvar intraepithelial neoplasia III. Gynecol Oncol 1990;37(1):34‐8. - PubMed
-
- Spirtos NM, Smith LH, Teng NNH. A prospective, randomized trial of topical interferon‐alpha (INF) gels for the treatment of vulvar intraepithelial neoplasia III (VIN III). Gynecological Oncology 1989; Vol. 32, issue 1:112, Abstract 76. - PubMed
Stier 2013 {published data only}
Todd 2005 {published data only}
-
- Todd RW, Luesley DM. Medical management of vulvar intraepithelial neoplasia. Journal of Lower Genital Tract Diseases 2005;9(4):206‐12. - PubMed
Van de Nieuwenhof 2008 {published data only}
-
- Nieuwenhof HP, Avoort IA, Massuger LF, Hullu JA. Letter to the Editor concerning "Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomized, double blinded study. Gynecological Oncology 2008;109(3):430‐1; author reply 431. - PubMed
References to ongoing studies
EUCTR2008‐008251‐42‐NL {published data only}
-
- Poelgeest M, Helmerhorst T. Clinical and immunological effects of imiquimod and HPV‐vaccination compared to imiquimod alone in female patients with Usual type VIN ‐ Effects of HPV‐vaccination and imiquimod in VIN patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... 2009:Accessed 06/02/2015.
EUCTR2011‐003134‐13‐NL {published data only}
-
- Blok L. 5‐Aminolevulinic acid photodynamic therapy for the treatment of pre‐malignant disorders of the vulva. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... 2011:Accessed 09/02/2015.
ISRCTN98495886 {published data only}
-
- Kaur B. Phase II clinical trial investigating the use of EPIgallocatechin‐3‐gallate (Veregen) in the treatment of Vulval Intraepithelial Neoplasia. http://www.isrctn.com/ISRCTN98495886 2013:Accessed 09/02/2015.
NCT01861535 {published data only}
-
- Trutnovsky G, Tamussino K. Primary imiquimod treatment versus surgery for vulvar intraepithelial neoplasia. https://clinicaltrials.gov/show/NCT01861535 2013:Accessed 09/02/15.
NTR1526 {published data only}
-
- Kenter G, Nijman HW. Randomised controlled study on the effects of imiquimod, a TLR 7 activating agent, on the HPV16‐specific immune response following HPV16 E6/E.7 synthetic long peptides vaccination in women with HPV16 positive high grade vulvar/vaginal intraepithelial neoplasia. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1526 2008:Accessed 09/02/2015.
Additional references
Aerts 2012
-
- Aerts L, Enzlin P, Vergote I, Verhaeghe J, Poppe W, Amant F. Sexual, psychological, and relational functioning in women after surgical treatment for vulvar malignancy: a literature review. Journal of Sexual Medicine 2012;9(2):361‐71. - PubMed
Andreasson 1986
-
- Andreasson B, Moth I, Jensen SB, Bock JE. Sexual function and somatopsychic reactions in vulvectomy‐operated women and their partners. Acta Obstetricia et Gynecologica Scandinavica 1986;65(1):7‐10. - PubMed
Baldwin 2003
-
- Baldwin PJ, Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia‐expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res 2003;9(14):5205‐13. - PubMed
BASHH guidelines 2014
-
- Clinical Effectiveness Unit of the British Association of Sexual Health and HIV. National guidelines on the management of vulval conditions. http://www.bashh.org/documents/UK%20national%20guideline%20for%20the%20m... 2014; Vol. Accessed 03/02/2015.
Bell 2000
-
- Bell MC, Crowley‐Nowick P, Bradlow HL, Sepkovic DW, Schmidt‐Grimminger D, Howell P, et al. Placebo‐Controlled Trial of Indole‐3‐Carbinol in the Treatment of CIN. Gynecologic Oncology 2000;78(2):123‐9. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. - PubMed
Couto 2014
Cox 2007
-
- Cox NH, Eedy DJ, Morton CA. Guidelines for the management of Bowen's disease: 2006 update. British Journal of Dermatology 2007;156:11‐21. - PubMed
CTCAE 2006
-
- CTCAE. Common terminology criteria for adverse events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Davidson 2003
-
- Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Research 2003;63(18):6032‐41. - PubMed
De Sanjose 2013
-
- Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. European Journal of Cancer 2013;49(16):3450‐61. - PubMed
De Vuyst 2009
-
- Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta‐analysis. International Journal of Cancer 2009 Apr 1;124(7):1626‐36. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Dittmer 2011
-
- Dittmer C, Katalinic A, Mundhenke C, Thill M, Fischer D. Epidemiology of vulvar and vaginal cancer in Germany. Archives of Gynecology and Obstetrics 2011;284(1):169‐74. - PubMed
Dominiak‐Felden 2013
-
- Dominiak‐Felden G, Cohet C, Atrux‐Tallau S, Gilet H, Tristram A, Fiander A. Impact of human papillomavirus‐related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health‐related quality of life study in the UK. BMC Public Health 2013;13:1065. - PMC - PubMed
Dougherty 1998
Eisenhauer 2009
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228‐47. - PubMed
Fehr 2001
-
- Fehr MK, Hornung R, Schwarz VA, Simeon R, Haller U, Wyss P. Photodynamic therapy of vulvar intraepithelial neoplasia III using topically applied 5‐aminolevulinic acid. Gynecologic Oncology 2001;80(1):62‐6. - PubMed
Fehr 2013
Fiander 2006
-
- Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime‐boost vaccination strategy in women with high‐grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. International Journal of Gynecological Cancer 2006;16(3):1075‐81. - PubMed
Foster 1981
-
- Foster DC, Woodruff JD. The use of dinitrochlorobenzene in the treatment of vulvar carcinoma in situ. Gynecological Oncology 1981 Jun;11(3):330‐9. - PubMed
Garrat 1995
-
- Garrat AM, Torgerson DJ, Wyness J, Hall MH, Reid DM. Measuring sexual function in premenopausal women. British Journal of Obstetrics and Gynaecolgy 1995;102:311‐5. - PubMed
GRADE Working Group 2004
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillemanns 2000
-
- Hillemanns P, Untch M, Dannecker C. Photodynamic therapy of vulvar intraepithelial neoplasia using 5‐aminolevulinic acid. International Journal of Cancer 2000;85(5):649‐53. - PubMed
ISSVD 2014
-
- ISSVD. 2014 Bibliography. Current ISSVD terminology. www.ISSVD.org 2014; Vol. http://issvd.org/wordpress/wp‐content/uploads/2014/02/2014‐BIBLIOGRAPHY‐....
Jones 1997
-
- Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstetrics and Gynecology 1997;90(3):448‐52. - PubMed
Jones 2005
-
- Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstetrics and Gynecology 2005 Dec;106(6):1319‐26. - PubMed
Joura 2000
-
- Joura EA, Lösch A, Haider‐Angeler MG, Breitenecker G, Leodolter S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. Journal of Reproductive Medicine 2000;45(8):613‐5. - PubMed
Judson 2006
-
- Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstetrics and Gynecology 2006;107(5):1018‐22. - PubMed
Kenter 2009
-
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends‐van der Meer DM, Vloon AP, et al. Vaccination against HPV‐16 oncoproteins for vulvar intraepithelial neoplasia. New England Journal of Medicine 2009;361(19):1838‐47. - PubMed
Lai 2014
-
- Lai J, Elleray R, Nordin A, Hirschowitz L, Rous B, Gildea C, et al. Vulval cancer incidence, mortality and survival in England: age‐related trends. BJOG .2014;121(6):728‐738. - PubMed
Lawrie 2015
Le 2007
-
- Le T, Menard C, Hicks‐Boucher W. Final results of a phase 2 study using continuous 5% Imiquimod cream application in the primary treatment of high‐grade vulva intraepithelial neoplasia. Gynecologic Oncology 2007;106(3):579‐84. - PubMed
McCluggage 2009
-
- McCluggage WG. Recent developments in vulvovaginal pathology. Histopathology 2009;54(2):156‐73. - PubMed
Miltz 2014
Moore 2001
Naransingh 2000
-
- Narayansingh GV, Cumming GP, Parkin DP, McConell DT, Honey E, Kolhe PS. Flap repair: an effective strategy for minimising sexual morbidity associated with the surgical management of vulval intraepithelial neoplasia. Journal of the Royal College of Surgeons of Edinburgh 2000;45(2):81‐4. - PubMed
Newfield 1998
-
- Newfield L, Bradlow HL, Sepkovic DW, Auborn K. Estrogen metabolism and the malignant potential of human papilloma virus immortalised keratinocytes. Proceedings of the Society for Experimental Biology and Medicine 1998;217:322‐6. - PubMed
Nygard 2014
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Preti 2015
Reyes 2014
-
- Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. Journal of Clinical Pathology 2014;67:290‐4. - PubMed
Roberts 1980
-
- Roberts JA, Watring WG, Lagasse LD. Treatment of vulvar intraepithelial neoplasia (VIN) with local bleomycin. Cancer Clinical Trials 1980;3(4):351‐4. - PubMed
Shylasree 2008
-
- Shylasree TS, Karanjgaokar V, Tristram A, Wilkes AR, MacLean AB, Fiander AN. Contribution of demographic, psychological and disease‐related factors to quality of life in women with high‐grade vulval intraepithelial neoplasia. Gynecologic Oncology 2008;110(2):185‐9. - PubMed
Sideri 2005
-
- Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. Journal of Reproductive Medicine 2005;50(11):807‐10. - PubMed
Sillman 1985
-
- Sillman FH, Sedlis A, Boyce JG. A review of lower genital intraepithelial neoplasia and the use of topical 5‐fluorouracil. Obstetrical and Gynecological Survey 1985;40(4):190‐220. - PubMed
Srodon 2006
-
- Srodon M, Stoler MH, Baber GB, Kurman RJ. The distribution of low and high‐risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). American Journal of Surgical Pathology 2006;12:1513‐8. - PubMed
Stanley 2010
Stern 2012
Terlou 2011
-
- Terlou A, Seters M, Ewing PC, Aaronson NK, Gundy CM, Heijmans‐Antonissen C, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: Seven years median follow‐up of a randomized clinical trial. Gynecologic Oncology 2011;121(1):157‐62. - PubMed
Todd 2002
-
- Todd RW, Etherington IJ, Leusley DM. The effects of 5% imiquimod on high‐grade vulval intraepithelial neoplasia. Gynecologic Oncology 2001;85:67‐70. - PubMed
Tristram 2005
-
- Tristram A, Fiander A. Clinical responses to cidofovir applied topically to women with high grade vulval intraepithelial neoplasia. Gynecologic Oncology 2005;99(3):652‐5. - PubMed
Van der Avoort 2006
-
- Avoort I, Shirango H, Hoevenaars BM, Grefte JM, Hullu JA, Wilde PC, et al. Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. International Journal of Gynecological Pathology 2006;25(1):22‐9. - PubMed
Van Esch 2012
-
- Esch EM, Welters MJ, Jordanova ES, Trimbos JB, Burg SH, Poelgeest MI. Treatment failure in patients with HPV 16‐induced vulvar intraepithelial neoplasia: understanding different clinical responses to immunotherapy. Expert Review of Vaccines 2012;11(7):821‐40. - PubMed
Van Esch 2013
-
- Esch EM, Dam MC, Osse ME, Putter H, Trimbos BJ, Fleuren G, et al. Clinical characteristics associated with development of recurrence and progression in usual‐type vulvar intraepithelial neoplasia. International Journal of Gynecological Cancer 2013;23(8):1476‐83. - PubMed
Van Esch 2015
-
- Esch EM, Poelgeest MI, Trimbos JB, Fleuren GJ, Jordanova ES, Burg SH. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV‐induced vulvar neoplasia. International Journal of Cancer 2015;136(4):E85‐94. - PubMed
Van Seters 2005
-
- Seters M, Beurden M, Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecologic Oncology 2005 May;97(2):645‐51. - PubMed
Wallbilich 2012
Wendling 2004
-
- Wendling J, Saiag P, Bervill‐Levy S, Bourgault‐Villada I, Clerici T, Moyal‐Barracco M. Treatment of undifferentiated vulvar intraepithelial neoplasia with 5% imiquimod cream. A prospective study of 12 cases. Archives of Dermatology 2004;140:1229‐4. - PubMed
Wilkinson 2014
-
- Wilkinson EJ, Cox JT, Selim MA, O'Connor DM. Evolution of terminology for human‐papillomavirus‐infection‐related vulvar squamous intraepithelial lesions. Journal of Lower Genital Tract Disease 2014;19(1):81‐7. - PubMed
Winters 2008
-
- Winters U, Daayana S, Lear JT, Tomilson AE, Elkord E, Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clinical Cancer Research 2008;14(16):5292‐99. - PubMed
Wu 2011
-
- Wu CH, Liang CH, Shiu LY, Chang LC, Lin TS, Lan CC, et al. Solanum incanum extract (SR‐T100) induces human cutaneous squamous cell carcinoma apoptosis through modulating tumor necrosis factor receptor signaling pathway. Journal of Dermatological Science 2011;63(2):83‐92. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

