Translating biosynthetic gene clusters into fungal armor and weaponry
- PMID: 26284674
- PMCID: PMC4682562
- DOI: 10.1038/nchembio.1897
Translating biosynthetic gene clusters into fungal armor and weaponry
Abstract
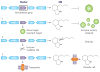
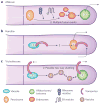
Filamentous fungi are renowned for the production of a diverse array of secondary metabolites (SMs) where the genetic material required for synthesis of a SM is typically arrayed in a biosynthetic gene cluster (BGC). These natural products are valued for their bioactive properties stemming from their functions in fungal biology, key among those protection from abiotic and biotic stress and establishment of a secure niche. The producing fungus must not only avoid self-harm from endogenous SMs but also deliver specific SMs at the right time to the right tissue requiring biochemical aid. This review highlights functions of BGCs beyond the enzymatic assembly of SMs, considering the timing and location of SM production and other proteins in the clusters that control SM activity. Specifically, self-protection is provided by both BGC-encoded mechanisms and non-BGC subcellular containment of toxic SM precursors; delivery and timing is orchestrated through cellular trafficking patterns and stress- and developmental-responsive transcriptional programs.
Conflict of interest statement
The author declares no competing financial interests.
Figures

References
-
- Demain AL, Fang A. The natural functions of secondary metabolites. Adv Biochem Eng Biotechnol. 2000;69:1–39. - PubMed
-
- Rohlfs M, Churchill AC. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet Biol. 2011;48:23–34. - PubMed
-
- Stergiopoulos I, Collemare J, Mehrabi R, De Wit PJ. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev. 2013;37:67–93. - PubMed
-
- Johnson JR, Bruce WF, Dutcher JD. Gliotoxin, the antibiotic principle of Gliocladium fimbriatum I Production, physical and biological properties. J Am Chem Soc. 1943;65:2005–2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

