HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- PMID: 26285145
- PMCID: PMC4540474
- DOI: 10.1371/journal.ppat.1005102
HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
Abstract
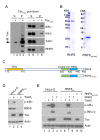
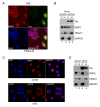
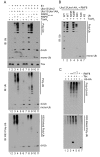
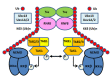
Human T lymphotropic virus type 1 (HTLV-1) trans-activator/oncoprotein, Tax, impacts a multitude of cellular processes, including I-κB kinase (IKK)/NF-κB signaling, DNA damage repair, and mitosis. These activities of Tax have been implicated in the development of adult T-cell leukemia (ATL) in HTLV-1-infected individuals, but the underlying mechanisms remain obscure. IKK and its upstream kinase, TGFβ-activated kinase 1 (TAK1), contain ubiquitin-binding subunits, NEMO and TAB2/3 respectively, which interact with K63-linked polyubiquitin (K63-pUb) chains. Recruitment to K63-pUb allows cross auto-phosphorylation and activation of TAK1 to occur, followed by TAK1-catalyzed IKK phosphorylation and activation. Using cytosolic extracts of HeLa and Jurkat T cells supplemented with purified proteins we have identified ubiquitin E3 ligase, ring finger protein 8 (RNF8), and E2 conjugating enzymes, Ubc13:Uev1A and Ubc13:Uev2, to be the cellular factors utilized by Tax for TAK1 and IKK activation. In vitro, the combination of Tax and RNF8 greatly stimulated TAK1, IKK, IκBα and JNK phosphorylation. In vivo, RNF8 over-expression augmented while RNF8 ablation drastically reduced canonical NF-κB activation by Tax. Activation of the non-canonical NF-κB pathway by Tax, however, is unaffected by the loss of RNF8. Using purified components, we further demonstrated biochemically that Tax greatly stimulated RNF8 and Ubc13:Uev1A/Uev2 to assemble long K63-pUb chains. Finally, co-transfection of Tax with increasing amounts of RNF8 greatly induced K63-pUb assembly in a dose-dependent manner. Thus, Tax targets RNF8 and Ubc13:Uev1A/Uev2 to promote the assembly of K63-pUb chains, which signal the activation of TAK1 and multiple downstream kinases including IKK and JNK. Because of the roles RNF8 and K63-pUb chains play in DNA damage repair and cytokinesis, this mechanism may also explain the genomic instability of HTLV-1-transformed T cells and ATL cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
RNF8 Dysregulation and Down-regulation During HTLV-1 Infection Promote Genomic Instability in Adult T-Cell Leukemia.PLoS Pathog. 2020 May 26;16(5):e1008618. doi: 10.1371/journal.ppat.1008618. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32453758 Free PMC article.
-
Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains.Proc Natl Acad Sci U S A. 2013 Sep 17;110(38):15247-52. doi: 10.1073/pnas.1314715110. Epub 2013 Aug 28. Proc Natl Acad Sci U S A. 2013. PMID: 23986494 Free PMC article.
-
HTLV-1 Tax Functions as a Ubiquitin E3 Ligase for Direct IKK Activation via Synthesis of Mixed-Linkage Polyubiquitin Chains.PLoS Pathog. 2016 Apr 15;12(4):e1005584. doi: 10.1371/journal.ppat.1005584. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27082114 Free PMC article.
-
HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ.Viruses. 2016 Jun 16;8(6):161. doi: 10.3390/v8060161. Viruses. 2016. PMID: 27322308 Free PMC article. Review.
-
Regulation of HTLV-1 tax stability, cellular trafficking and NF-κB activation by the ubiquitin-proteasome pathway.Viruses. 2014 Oct 23;6(10):3925-43. doi: 10.3390/v6103925. Viruses. 2014. PMID: 25341660 Free PMC article. Review.
Cited by
-
NF-κB-Induced R-Loops and Genomic Instability in HTLV-1-Infected and Adult T-Cell Leukemia Cells.Viruses. 2022 Apr 23;14(5):877. doi: 10.3390/v14050877. Viruses. 2022. PMID: 35632619 Free PMC article. Review.
-
NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma.FEBS J. 2018 Sep;285(18):3324-3336. doi: 10.1111/febs.14492. Epub 2018 May 14. FEBS J. 2018. PMID: 29722927 Free PMC article. Review.
-
The TP53-Induced Glycolysis and Apoptosis Regulator mediates cooperation between HTLV-1 p30II and the retroviral oncoproteins Tax and HBZ and is highly expressed in an in vivo xenograft model of HTLV-1-induced lymphoma.Virology. 2018 Jul;520:39-58. doi: 10.1016/j.virol.2018.05.007. Epub 2018 May 26. Virology. 2018. PMID: 29777913 Free PMC article.
-
RNF8 Dysregulation and Down-regulation During HTLV-1 Infection Promote Genomic Instability in Adult T-Cell Leukemia.PLoS Pathog. 2020 May 26;16(5):e1008618. doi: 10.1371/journal.ppat.1008618. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32453758 Free PMC article.
-
The E3/E4 ubiquitin conjugation factor UBE4B interacts with and ubiquitinates the HTLV-1 Tax oncoprotein to promote NF-κB activation.PLoS Pathog. 2020 Dec 23;16(12):e1008504. doi: 10.1371/journal.ppat.1008504. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33362245 Free PMC article.
References
-
- Chu ZL, Shin YA, Yang JM, Di Donato JA, Ballard DW LH (1999) IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. JBiolChem 1999 May 28;274: 15297–15300. - PubMed
-
- Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT LH (1999) Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. JBiolChem 1999 June 18;274: 17402–17405. - PubMed
-
- Xiao G, Sun SC (2000) Activation of IKKalpha and IKKbeta through their fusion with HTLV-I tax protein. Oncogene 2000 October 26;19: 5198–5203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

