Long-term safety and efficacy of antithymocyte globulin induction: use of integrated national registry data to achieve ten-year follow-up of 10-10 Study participants
- PMID: 26285695
- PMCID: PMC4545548
- DOI: 10.1186/s13063-015-0891-y
Long-term safety and efficacy of antithymocyte globulin induction: use of integrated national registry data to achieve ten-year follow-up of 10-10 Study participants
Erratum in
-
Erratum to: Long-term safety and efficacy of antithymocyte globulin induction: use of integrated national registry data to achieve ten-year follow-up of 10-10 Study participants.Trials. 2015 Sep 16;16:412. doi: 10.1186/s13063-015-0940-6. Trials. 2015. PMID: 26377405 Free PMC article. No abstract available.
Abstract
Background: Rabbit antithymocyte globulin (rATG, Thymoglobulin®) is the most common induction immunosuppression therapy in kidney transplantation. We applied a database integration strategy to capture and compare long-term (10-year) outcome data for US participants in a clinical trial of rATG versus FDA-approved basiliximab.
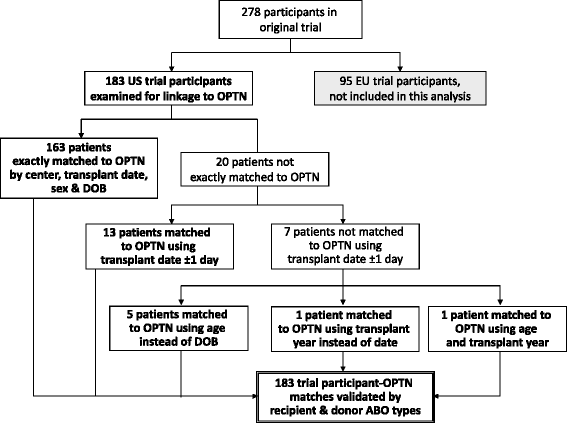
Methods: Records for US participants in an international, 1-year, randomized clinical trial comparing rATG and basiliximab induction in deceased donor kidney transplantation were integrated with records from the US national Organ Procurement and Transplantation (OPTN) registry using center, transplant dates, recipient sex, and birthdates. The OPTN captures center-reported acute rejection, graft failure, death, and cancer events, and incorporates comprehensive death records from the Social Security Death Master File. Ten-year outcomes according to randomized induction regimen were compared by Kaplan-Meier analysis (two-sided P). Non-inferiority of rATG was assessed using a one-tailed equivalence test (a-priori equivalence margins of 0-10 %).
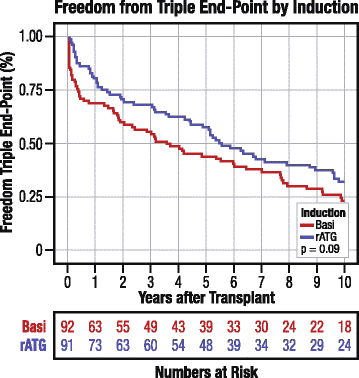
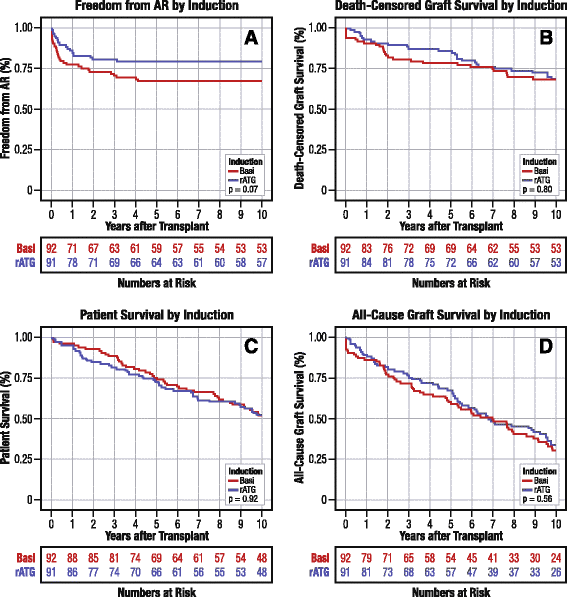
Results: Of 183 US trial participants, 89 % (n = 163) matched OPTN records exactly; the remainder were matched by extending agreement windows for transplant and birthdates. Matches were validated by donor and recipient blood types. By Kaplan-Meier analysis, 10 years post-transplant, freedom from acute rejection, graft failure, or death was 32.6 % and 24.0 % in the rATG and basiliximab arms, respectively (P = 0.09). The incidence of acute rejection with rATG versus basiliximab induction was 21.0 % versus 32.8 % (P = 0.07). Patient survival (52.8 % [Corrected] versus 52.2 %, P = 0.92) and graft survival (34.3 % versus 30.9 %, P = 0.56) rates were numerically and statistically similar for both arms. Comparison of the composite outcome meets non-inferiority criteria even with a 0 % equivalence margin (one-sided P = 0.04). With a 10 % equivalence margin, the odds that rATG is no worse than basiliximab for 10-year risk of the composite endpoint are >99 %.
Conclusions: Ten years post-transplant, rATG induction has comparable efficacy and safety to FDA-approved basiliximab. Integration of clinical trial records with national registry data can enable long-term monitoring of trial participants in transplantation, circumventing logistical and cost barriers of extended follow-up.
Trial registration: ClinicalTrials.gov NCT00235300.
Figures
Similar articles
-
Rabbit antithymocyte globulin versus basiliximab in renal transplantation.N Engl J Med. 2006 Nov 9;355(19):1967-77. doi: 10.1056/NEJMoa060068. N Engl J Med. 2006. PMID: 17093248 Clinical Trial.
-
Efficacy and safety of thymoglobulin and basiliximab in kidney transplant patients at high risk for acute rejection and delayed graft function.Exp Clin Transplant. 2013 Aug;11(4):310-4. doi: 10.6002/ect.2012.0103. Epub 2012 Nov 1. Exp Clin Transplant. 2013. PMID: 23121641
-
Low-dose RATG with or without basiliximab in renal transplantation: a matched-cohort observational study.Am J Nephrol. 2015;41(1):16-27. doi: 10.1159/000371728. Epub 2015 Jan 23. Am J Nephrol. 2015. PMID: 25612603
-
Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection.Drugs. 2009 Jul 30;69(11):1483-512. doi: 10.2165/00003495-200969110-00007. Drugs. 2009. PMID: 19634926 Review.
-
Basiliximab: a review of its use as induction therapy in renal transplantation.Drugs. 2003;63(24):2803-35. doi: 10.2165/00003495-200363240-00009. Drugs. 2003. PMID: 14664658 Review.
Cited by
-
The Risk of Postkidney Transplant Outcomes by Induction Choice Differs by Recipient Age.Transplant Direct. 2021 Jun 18;7(7):e715. doi: 10.1097/TXD.0000000000001105. eCollection 2021 Jul. Transplant Direct. 2021. PMID: 34476294 Free PMC article.
-
Induction therapy in kidney transplant recipients: Description of the practices according to the calendar period from the French multicentric DIVAT cohort.PLoS One. 2020 Oct 22;15(10):e0240929. doi: 10.1371/journal.pone.0240929. eCollection 2020. PLoS One. 2020. PMID: 33091057 Free PMC article.
-
Graft Pre-conditioning by Peri-Operative Perfusion of Kidney Allografts With Rabbit Anti-human T-lymphocyte Globulin Results in Improved Kidney Graft Function in the Early Post-transplantation Period-a Prospective, Randomized Placebo-Controlled Trial.Front Immunol. 2018 Aug 24;9:1911. doi: 10.3389/fimmu.2018.01911. eCollection 2018. Front Immunol. 2018. PMID: 30197644 Free PMC article. Clinical Trial.
-
Demonstrating Benefit-Risk Profiles of Novel Therapeutic Strategies in Kidney Transplantation: Opportunities and Challenges of Real-World Evidence.Transpl Int. 2022 May 3;35:10329. doi: 10.3389/ti.2022.10329. eCollection 2022. Transpl Int. 2022. PMID: 35592446 Free PMC article.
-
Renal association clinical practice guideline in post-operative care in the kidney transplant recipient.BMC Nephrol. 2017 Jun 2;18(1):174. doi: 10.1186/s12882-017-0553-2. BMC Nephrol. 2017. PMID: 28571571 Free PMC article.
References
-
- Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation. 2003;76(9):1289–93. doi: 10.1097/01.TP.0000100826.58738.2B. - DOI - PubMed
-
- United States Renal Data System (USRDS). 2014 Annual Data Report. Chapter 6: Transplantation. Available at: http://www.usrds.org/2014/view/v2_06.aspx. Accessed 1 July 2015.
-
- Administration USFaD. 1998 Approval Letter – Thymoglobulin. http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedPro.... Accessed 1 July 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

