Tissue-engineered tubular substitutions for urinary diversion in a rabbit model
- PMID: 26286106
- PMCID: PMC4935384
- DOI: 10.1177/1535370215600101
Tissue-engineered tubular substitutions for urinary diversion in a rabbit model
Abstract
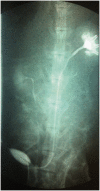
Clinically, autologous gastrointestinal segments are traditionally used for urinary diversion. However, this procedure often causes many serious complications. Tissue engineering may provide an alternative treatment method in urinary diversion. This research aims to produce tissue-engineered tubular substitutions by using homologous adipose-derived stem cells, smooth muscle cells, and bladder acellular matrix in developing urinary diversion in a rabbit model. Adipose-derived stem cells and smooth muscle cells of rabbit were obtained and cultured in vitro. These cultured adipose-derived stem cells and smooth muscle cells were seeded onto the two sides of the bladder acellular matrix and then incubated for seven days. The cell-seeded matrix was used to build tissue-engineered tubular substitutions, which were then implanted and wrapped into the omentum in vivo for two weeks to promote angiogenesis. In the experimental group, the bladder of 20 rabbits was totally resected, and the above tissue-engineered tubular substitutions were used for urinary diversion. In the control group, bladder acellular matrix tubular substitutions with unseeded cells were implanted into the omentum and were used as urinary diversion on another five rabbits with the same process. The implants were harvested, and histological examination was conducted at 2, 4, 8, and 16 weeks after operation. Intravenous urography assessment was performed at 16 weeks postoperatively. All the rabbits were alive in the experimental group until they were sacrificed. Histological analysis of the construct displayed the presence of multilayer urothelial cells on the luminal side and organized smooth muscle tissue on the other side, and different diameters of neovascularization were clearly identified in the substitutions obtained. No leakage, stricture, or obstructions were noted with intravenous urography assessment. All the animals in the control group died within two weeks, and urine leakage, scar formation, and inflammation were detected through autopsy. This study demonstrates the feasibility of tissue-engineered tubular substitutions constructed using homologous adipose-derived stem cells, smooth muscle cells, and bladder acellular matrix for urinary diversion in a rabbit model.
Keywords: Tissue engineering; adipose-derived stem cells; bladder acellular matrix; epithelium; omentum; smooth muscle cells; urinary diversion.
© 2015 by the Society for Experimental Biology and Medicine.
Figures







References
-
- Biers SM, Venn SN, Greenwell TJ. The past, present and future of augmentation cystoplasty. BJU Int 2012; 109: 1280–93. - PubMed
-
- Scales CD, Jr, Wiener JS. Evaluating outcomes of enterocystoplasty in patients with spina bifida: a review of the literature. J Urol 2008; 180: 2323–9. - PubMed
-
- Husmann DA, Rathbun SR. Long-term follow up of enteric bladder augmentations: the risk for malignancy. J Pediatr Urol 2008; 4: 381–5; discussion 6. - PubMed
-
- Zhang Y, Atala A. Urothelial cell culture: stratified urothelial sheet and three-dimensional growth of urothelial structure. Methods Mol Biol 2013; 945: 383–99. - PubMed
-
- Horst M, Madduri S, Gobet R, Sulser T, Milleret V, Hall H, Atala A, Eberli D. Engineering functional bladder tissues. J Tissue Eng Regener Med 2013; 7: 515–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

