Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial
- PMID: 26286141
- PMCID: PMC4545560
- DOI: 10.1186/s12890-015-0092-1
Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial
Abstract
Background: Umeclidinium (UMEC; long-acting muscarinic antagonist) plus vilanterol (VI; long-acting beta2 agonist [LABA]) and the LABA/inhaled corticosteroid fluticasone propionate/salmeterol (FP/SAL) are approved maintenance treatments for chronic obstructive pulmonary disease (COPD). This 12-week, multicentre, double-blind, parallel-group, double-dummy study compared the efficacy and safety of these treatments in symptomatic patients with moderate-to-severe COPD with no exacerbations in the year prior to enrolment.
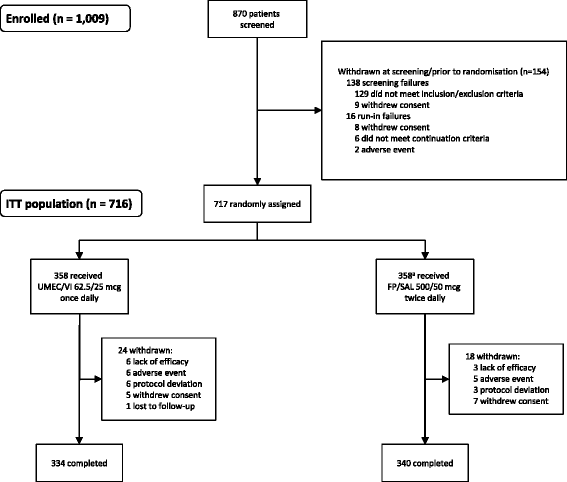
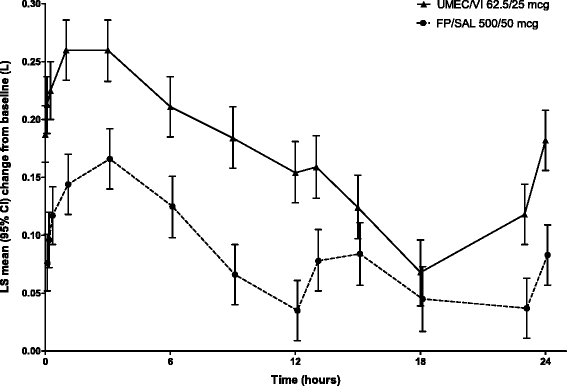
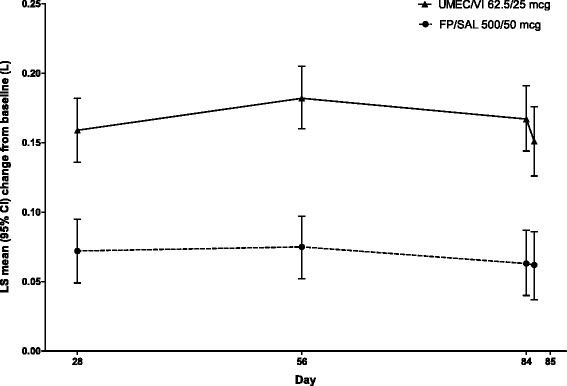
Methods: Patients (n = 717) were randomised 1:1 to once-daily UMEC/VI 62.5/25 mcg or twice-daily FP/SAL 500/50 mcg. Endpoints included 0-24 h weighted mean (wm) forced expiratory volume in 1 s (FEV1) (Day 84; primary), trough FEV1 (Day 85; secondary), other lung function endpoints, symptoms, quality of life (QoL) and safety.
Results: Improvements with UMEC/VI versus FP/SAL were 0.080 L (95 % confidence interval: 0.046-0.113; wmFEV1) and 0.090 L (0.055-0.125; trough FEV1) (both p < 0.001). UMEC/VI statistically significantly improved all other lung function measures versus FP/SAL. Both treatments demonstrated a clinically meaningful improvement in symptoms (Transition Dyspnoea Index ≥1 unit) and QoL (St George's Respiratory Questionnaire Total score ≥4 unit decrease from baseline) over 12 weeks. The incidence of adverse events was 28 % (UMEC/VI) and 29 % (FP/SAL); nasopharyngitis and headache were most common.
Conclusions: Once-daily UMEC/VI 62.5/25 mcg over 12 weeks resulted in significant and sustained improvements in lung function versus twice-daily FP/SAL 500/50 mcg in patients with moderate-to-severe COPD and with no exacerbations in the year prior to enrolment.
Trial registration: NCT01822899 Registration date: March 28, 2013.
Figures
References
-
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-managem... (2014). Accessed 17 Dec 2014.
-
- Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, Rossi A, Hutton C, Ashton VL, Stewart R, Bichel K. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–905. doi: 10.2147/COPD.S62750. - DOI - PMC - PubMed
-
- Anoro EU summary of product characteristics, 16 May 2014. http://www.medicines.ie/medicine/16007/SPC/ANORO+55+micrograms+22+microg... (2014). Accessed 17 Dec 2014.
-
- ANORO US prescribing information, May 2014. https://www.gsksource.com/gskprm/htdocs/documents/ANORO-ELLIPTA-PI-MG.PDF (2014). Accessed 17 Dec 2014.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

