The Evaluation of the Therapeutic Efficacy and Side Effects of a Macromolecular Dexamethasone Prodrug in the Collagen-Induced Arthritis Mouse Model
- PMID: 26286188
- PMCID: PMC4690754
- DOI: 10.1007/s11095-015-1776-1
The Evaluation of the Therapeutic Efficacy and Side Effects of a Macromolecular Dexamethasone Prodrug in the Collagen-Induced Arthritis Mouse Model
Abstract
Purpose: To investigate the efficacy and safety of N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-dexamethasone conjugate (P-Dex) in the collagen-induced arthritis (CIA) mouse model.
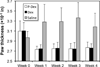
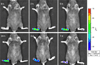
Methods: HPMA copolymer labeled with a near infrared fluorescence (NIRF) dye was administered to mice with CIA to validate its passive targeting to inflamed joints and utility as a drug carrier system. The CIA mice were treated with P-Dex, dexamethasone (Dex) or saline and the therapeutic efficacy and skeletal toxicity evaluated using clinical scoring and micro-computed tomography (μ-CT).
Results: The NIRF signal of the HPMA copolymer localized to arthritic joints consistent with its passive targeting to sites of inflammation. While the CIA mice responded more rapidly to P-Dex compared to Dex, the final clinical score and endpoint μ-CT analyses of localized bone erosions indicated that both single dose P-Dex and dose equivalent daily Dex led to comparable clinical efficacy after 30 days. μ-CT analysis of the proximal tibial metaphyses showed that P-Dex treatment was associated with significantly higher BMD and BV/TV compared to Dex and the saline control, consistent with reduced glucocorticoid (GC) skeletal toxicity.
Conclusion: These results validate the therapeutic efficacy of P-Dex in the CIA mouse model. P-Dex treatment averted the adverse effects of GC's on systemic bone loss, supporting its utility in clinical development for the management of rheumatoid arthritis.
Keywords: ELVIS; HPMA copolymer; collagen-induced arthritis; glucocorticoid; prodrug.
Figures


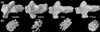

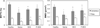
Similar articles
-
Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy.Arthritis Res Ther. 2010;12(5):R170. doi: 10.1186/ar3130. Epub 2010 Sep 13. Arthritis Res Ther. 2010. PMID: 20836843 Free PMC article.
-
Macromolecular prodrug of dexamethasone prevents particle-induced peri-implant osteolysis with reduced systemic side effects.J Control Release. 2014 Feb 10;175:1-9. doi: 10.1016/j.jconrel.2013.11.024. Epub 2013 Dec 8. J Control Release. 2014. PMID: 24326124 Free PMC article.
-
Effect of dexamethasone prodrug on inflamed temporomandibular joints in juvenile rats.Arthritis Res Ther. 2015 Sep 24;17(1):267. doi: 10.1186/s13075-015-0772-5. Arthritis Res Ther. 2015. PMID: 26400235 Free PMC article.
-
Development of macromolecular prodrug for rheumatoid arthritis.Adv Drug Deliv Rev. 2012 Sep;64(12):1205-19. doi: 10.1016/j.addr.2012.03.006. Epub 2012 Mar 10. Adv Drug Deliv Rev. 2012. PMID: 22433784 Free PMC article. Review.
-
Dexamethasone: chondroprotective corticosteroid or catabolic killer?Eur Cell Mater. 2019 Nov 22;38:246-263. doi: 10.22203/eCM.v038a17. Eur Cell Mater. 2019. PMID: 31755076 Free PMC article. Review.
Cited by
-
Structural optimization of HPMA copolymer-based dexamethasone prodrug for improved treatment of inflammatory arthritis.J Control Release. 2020 Aug 10;324:560-573. doi: 10.1016/j.jconrel.2020.05.028. Epub 2020 May 20. J Control Release. 2020. PMID: 32445658 Free PMC article.
-
Polymer nanomedicines.Adv Drug Deliv Rev. 2020;156:40-64. doi: 10.1016/j.addr.2020.07.020. Epub 2020 Jul 28. Adv Drug Deliv Rev. 2020. PMID: 32735811 Free PMC article. Review.
-
Exploration and Evaluation of Therapeutic Efficacy of Drug-Free Macromolecular Therapeutics in Collagen-Induced Rheumatoid Arthritis Mouse Model.Macromol Biosci. 2020 May;20(5):e1900445. doi: 10.1002/mabi.201900445. Epub 2020 Mar 20. Macromol Biosci. 2020. PMID: 32196951 Free PMC article.
-
Osteoclast-Derived Autotaxin, a Distinguishing Factor for Inflammatory Bone Loss.Arthritis Rheumatol. 2019 Nov;71(11):1801-1811. doi: 10.1002/art.41005. Epub 2019 Sep 30. Arthritis Rheumatol. 2019. PMID: 31162832 Free PMC article.
-
Pharmacokinetic and Biodistribution Studies of HPMA Copolymer Conjugates in an Aseptic Implant Loosening Mouse Model.Mol Pharm. 2017 May 1;14(5):1418-1428. doi: 10.1021/acs.molpharmaceut.7b00045. Epub 2017 Apr 5. Mol Pharm. 2017. PMID: 28343392 Free PMC article.
References
-
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009 Feb 21;373(9664):659–672. PubMed PMID: 19157532. - PubMed
-
- van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nature reviews Rheumatology. 2009 Oct;5(10):531–541. PubMed PMID: 19798027. - PubMed
-
- Hoes JN, Jacobs JW, Buttgereit F, Bijlsma JW. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat Rev Rheumatol. 2010 Dec;6(12):693–702. PubMed PMID: 21119718. - PubMed
-
- Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, et al. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharmaceutical research. 2008 Dec;25(12):2910–9. PubMed PMID: 18649124. Pubmed Central PMCID: 2593120. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

