Immobilization of trypsin in organic and aqueous media for enzymatic peptide synthesis and hydrolysis reactions
- PMID: 26286267
- PMCID: PMC4545374
- DOI: 10.1186/s12896-015-0196-y
Immobilization of trypsin in organic and aqueous media for enzymatic peptide synthesis and hydrolysis reactions
Abstract
Background: Immobilization of enzymes onto different carriers increases enzyme's stability and reusability within biotechnological and pharmaceutical applications. However, some immobilization techniques are associated with loss of enzymatic specificity and/or activity. Possible reasons for this loss are mass transport limitations or structural changes. For this reason an immobilization method must be selected depending on immobilisate's demands. In this work different immobilization media were compared towards the synthetic and hydrolytic activities of immobilized trypsin as model enzyme on magnetic micro-particles.
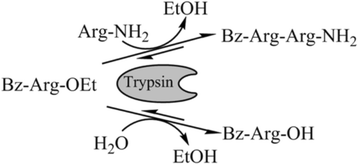
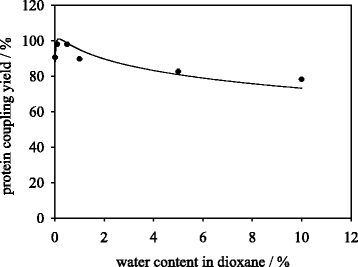
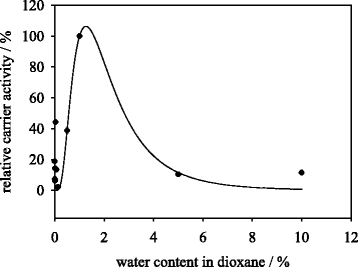
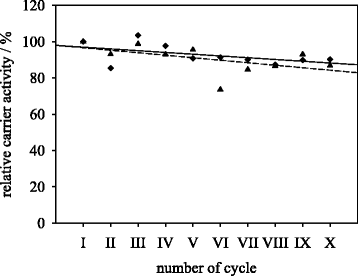
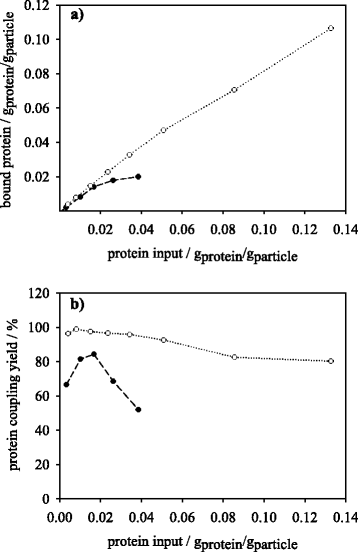
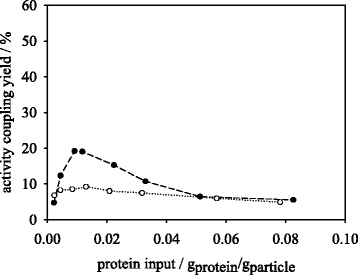
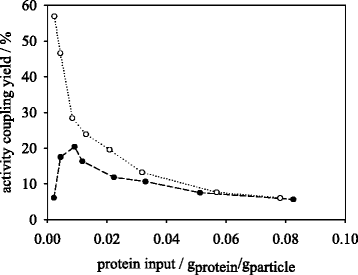
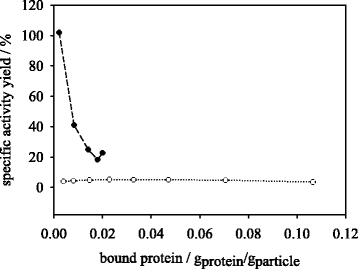
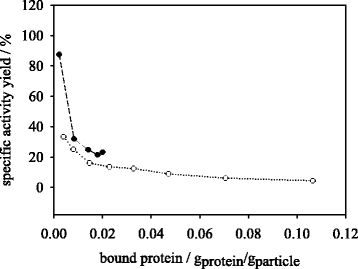
Results: Porcine trypsin immobilization was carried out in organic and aqueous media with magnetic microparticles. The immobilization conditions in organic solvent were optimized for a peptide synthesis reaction. The highest carrier activity was achieved at 1 % of water (v/v) in dioxane. The resulting immobilizate could be used over ten cycles with activity retention of 90 % in peptide synthesis reaction in 80 % (v/v) ethanol and in hydrolysis reaction with activity retention of 87 % in buffered aqueous solution. Further, the optimized method was applied in peptide synthesis and hydrolysis reactions in comparison to an aqueous immobilization method varying the protein input. The dioxane immobilization method showed a higher activity coupling yield by factor 2 in peptide synthesis with a maximum activity coupling yield of 19.2 % compared to aqueous immobilization. The hydrolysis activity coupling yield displayed a maximum value of 20.4 % in dioxane immobilization method while the aqueous method achieved a maximum value of 38.5 %. Comparing the specific activity yields of the tested immobilization methods revealed maximum values of 5.2 % and 100 % in peptide synthesis and 33.3 % and 87.5 % in hydrolysis reaction for the dioxane and aqueous method, respectively.
Conclusions: By immobilizing trypsin in dioxane, a beneficial effect on the synthetic trypsin activity resilience compared to aqueous immobilization medium was shown. The results indicate a substantial potential of the micro-aqueous organic protease immobilization method for preservation of enzymatic activity during enzyme coupling step. These results may be of substantial interest for enzymatic peptide synthesis reactions at mild conditions with high selectivity in industrial drug production.
Figures
References
-
- Cao L. Carrier-bound immobilized enzymes. Weinheim: Wiley-VCH; 2006.
-
- Buchholz K, Kasche V, Bornscheuer UT. Biocatalysts and enzyme technology. Weinheim: Wiley-VCH; 2005. pp. 338–343.
-
- Halling PJ, Dunnill P. Magnetic supports for immobilized enzymes and bioaffinity adsorbents. Enzym Microb Technol. 1980;2(1):2–10. doi: 10.1016/0141-0229(80)90002-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

