Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents
- PMID: 26286657
- PMCID: PMC4561402
- DOI: 10.1101/lm.037267.114
Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents
Abstract
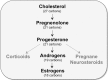
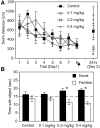
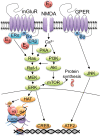
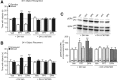
Ample evidence has demonstrated that sex steroid hormones, such as the potent estrogen 17β-estradiol (E2), affect hippocampal morphology, plasticity, and memory in male and female rodents. Yet relatively few investigators who work with male subjects consider the effects of these hormones on learning and memory. This review describes the effects of E2 on hippocampal spinogenesis, neurogenesis, physiology, and memory, with particular attention paid to the effects of E2 in male rodents. The estrogen receptors, cell-signaling pathways, and epigenetic processes necessary for E2 to enhance memory in female rodents are also discussed in detail. Finally, practical considerations for working with female rodents are described for those investigators thinking of adding females to their experimental designs.
© 2015 Frick et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
