Life-history plasticity in female threespine stickleback
- PMID: 26286665
- PMCID: PMC4815461
- DOI: 10.1038/hdy.2015.65
Life-history plasticity in female threespine stickleback
Abstract
The postglacial adaptive radiation of the threespine stickleback fish (Gasterosteus aculeatus) has been widely used to investigate the roles of both adaptive evolution and plasticity in behavioral and morphological divergence from the ancestral condition represented by present-day oceanic stickleback. These phenotypes tend to exhibit high levels of ecotypic differentiation. Population divergence in life history has also been well studied, but in contrast to behavior and morphology, the extent and importance of plasticity has been much less well studied. In this review, we summarize what is known about life-history plasticity in female threespine stickleback, considering four traits intimately associated with reproductive output: age/size at maturation, level of reproductive effort, egg size and clutch size. We envision life-history plasticity in an iterative, ontogenetic framework, in which females may express plasticity repeatedly across each of several time frames. We contrast the results of laboratory and field studies because, for most traits, these approaches give somewhat different answers. We provide ideas on what the cues might be for observed plasticity in each trait and, when possible, we inquire about the relative costs and benefits to expressed plasticity. We end with an example of how we think plasticity may play out in stickleback life history given what we know of plasticity in the ancestor.
Figures
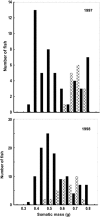
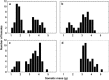
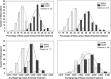
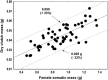





References
-
- Adolph SC, Porter PP. (1996). Growth, seasonality, and lizard life histories: age and size at maturity. Oikos 77: 267–278.
-
- Ali M, Wootton RJ. (1999. a). Coping with resource variation: effect of constant and variable intervals between feeding on reproductive performance at first spawning of female three-spined sticklebacks. J Fish Biol 55: 211–220.
-
- Ali M, Wootton RJ. (1999. b). Effect of variable food levels on reproductive performance of breeding female three-spined sticklebacks. J Fish Biol 55: 1040–1053.
-
- Allen RM, Buckley YM, Marshall DJ. (2008). Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am Nat 171: 225–237. - PubMed
-
- Anderson JT. (1988). A review of size dependent survival during pre-recruit stages of fish in relation to recruitment. J Northwest Atl Fish Sci 8: 55–66.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

