Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose)
- PMID: 26286827
- PMCID: PMC4560800
- DOI: 10.1038/ncomms9088
Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose)
Abstract
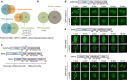
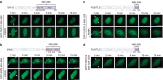
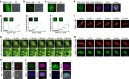
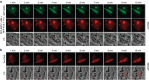
Intrinsically disordered proteins can phase separate from the soluble intracellular space, and tend to aggregate under pathological conditions. The physiological functions and molecular triggers of liquid demixing by phase separation are not well understood. Here we show in vitro and in vivo that the nucleic acid-mimicking biopolymer poly(ADP-ribose) (PAR) nucleates intracellular liquid demixing. PAR levels are markedly induced at sites of DNA damage, and we provide evidence that PAR-seeded liquid demixing results in rapid, yet transient and fully reversible assembly of various intrinsically disordered proteins at DNA break sites. Demixing, which relies on electrostatic interactions between positively charged RGG repeats and negatively charged PAR, is amplified by aggregation-prone prion-like domains, and orchestrates the earliest cellular responses to DNA breakage. We propose that PAR-seeded liquid demixing is a general mechanism to dynamically reorganize the soluble nuclear space with implications for pathological protein aggregation caused by derailed phase separation.
Figures

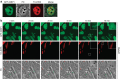
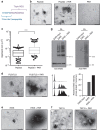
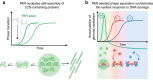
Comment in
-
PAR and the organization of the DNA damage response.Nat Struct Mol Biol. 2015 Sep;22(9):655. doi: 10.1038/nsmb.3089. Nat Struct Mol Biol. 2015. PMID: 26333714 No abstract available.
References
-
- Malinovska L., Kroschwald S. & Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim. Biophys. Acta. 1834, 918–931 (2013). - PubMed
-
- Han T. W. et al.. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

