Genome Instability in Development and Aging: Insights from Nucleotide Excision Repair in Humans, Mice, and Worms
- PMID: 26287260
- PMCID: PMC4598778
- DOI: 10.3390/biom5031855
Genome Instability in Development and Aging: Insights from Nucleotide Excision Repair in Humans, Mice, and Worms
Abstract
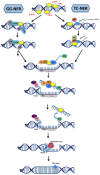
DNA damage causally contributes to aging and cancer. Congenital defects in nucleotide excision repair (NER) lead to distinct cancer-prone and premature aging syndromes. The genetics of NER mutations have provided important insights into the distinct consequences of genome instability. Recent work in mice and C. elegans has shed new light on the mechanisms through which developing and aging animals respond to persistent DNA damage. The various NER mouse mutants have served as important disease models for Xeroderma pigmentosum (XP), Cockayne syndrome (CS), and trichothiodystrophy (TTD), while the traceable genetics of C. elegans have allowed the mechanistic delineation of the distinct outcomes of genome instability in metazoan development and aging. Intriguingly, highly conserved longevity assurance mechanisms respond to transcription-blocking DNA lesions in mammals as well as in worms and counteract the detrimental consequences of persistent DNA damage. The insulin-like growth factor signaling (IIS) effector transcription factor DAF-16 could indeed overcome DNA damage-driven developmental growth delay and functional deterioration even when DNA damage persists. Longevity assurance mechanisms might thus delay DNA damage-driven aging by raising the threshold when accumulating DNA damage becomes detrimental for physiological tissue functioning.
Keywords: Cockayne syndrome (CS); DNA damage; Global-genome nucleotide-excision repair (GG-NER); Nucleotide-excision repair (NER); Transcription-coupled nucleotide excision repair (TC-NER); Ultraviolet Light (UV); aging; growth hormone/insulin-like growth factor 1 (GH/IGF1) signaling; longevity; somatotropic axis.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

