Fluctuating Behavior and Influential Factors in the Performance of the QuantiFERON-TB Gold In-Tube Assay in the Diagnosis of Tuberculosis
- PMID: 26287382
- PMCID: PMC4545827
- DOI: 10.1371/journal.pone.0103763
Fluctuating Behavior and Influential Factors in the Performance of the QuantiFERON-TB Gold In-Tube Assay in the Diagnosis of Tuberculosis
Abstract
Background: The QuantiFERON-TB Gold In-Tube (QFT-GIT) is a newly developed but widely used interferon-γ release assay for diagnosing tuberculosis (TB). However, research has not determined whether age or the use of an immune suppressive or anti-TB treatment influences this assay's ability to detect TB. We assessed the QFT-GIT diagnostic performance for active tuberculosis (ATB) in children and adults in an endemic country and explored the effects of glucocorticoids and anti-TB therapy on the diagnostic value of the QFT-GIT.
Methods: A total of 60 children and 212 adults with suspected ATB were evaluated with the QFT-GIT. The association between the QFT-GIT diagnostic value and pretreatment factors was qualitatively and quantitatively assessed.
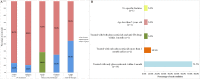
Results: The sensitivity of the QFT-GIT was 83.9% (95% CI 66.3%-94.6%) in children, and 73.7% (95% CI 57.8%-85.2%) in adults. Glucocorticoids affected the mitogen-stimulated response in both children and adults. In subjects undergoing glucocorticoid pretreatment, 25.0% of the children presented with false-negative QFT-GIT results, 28.6% of adults presented with indeterminate results. For subjects pre-treated with anti-TB drugs, 44.4% presented with false-negative QFT-GIT results.
Conclusions: The QFT-GIT has higher sensitivity and specificity in children than adults. Glucocorticoid treatment negatively impacts the diagnostic value of the QFT-GIT in all age groups. Anti-TB treatment decreases the sensitivity of the QFT-GIT. Therefore, we recommend that the QFT-GIT assay be performed before TB-specific treatment is initiated and the test should not be used on people undergoing immunosuppression treatment, regardless of their age. A quantitative analysis of the QFT-GIT could be useful for assessing and monitoring TB-specific and non-specific immunity during conversion of the disease.
Conflict of interest statement
Figures


Similar articles
-
[The evaluation of the utility of QuantiFERON TB-Gold In-Tube; QFT-GIT].Kekkaku. 2014 Sep;89(9):743-55. Kekkaku. 2014. PMID: 25730946 Japanese.
-
Diagnostic usefulness of the QuantiFERON-TB gold in-tube test (QFT-GIT) for tuberculous vertebral osteomyelitis.Infect Dis (Lond). 2018 May;50(5):346-351. doi: 10.1080/23744235.2017.1410282. Epub 2017 Nov 30. Infect Dis (Lond). 2018. PMID: 29189087
-
QuantiFERON®-TB Gold In-Tube assay vs. tuberculin skin test in Indonesian children living with a tuberculosis case.Int J Tuberc Lung Dis. 2012 Apr;16(4):496-502. doi: 10.5588/ijtld.11.0491. Int J Tuberc Lung Dis. 2012. PMID: 22325792
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon-γ release assays: A systematic review and meta-analysis.Adv Med Sci. 2019 Sep;64(2):437-443. doi: 10.1016/j.advms.2019.09.001. Epub 2019 Oct 3. Adv Med Sci. 2019. PMID: 31586819
Cited by
-
Immune-mediated inflammatory diseases differently affect IGRAs' accuracy for latent tuberculosis infection diagnosis in clinical practice.PLoS One. 2017 Dec 7;12(12):e0189202. doi: 10.1371/journal.pone.0189202. eCollection 2017. PLoS One. 2017. PMID: 29216277 Free PMC article.
-
Risk Factors for Indeterminate Interferon-Gamma Release Assay for the Diagnosis of Tuberculosis in Children-A Systematic Review and Meta-Analysis.Front Pediatr. 2019 May 29;7:208. doi: 10.3389/fped.2019.00208. eCollection 2019. Front Pediatr. 2019. PMID: 31192175 Free PMC article.
-
Role of interferon gamma release assay in the diagnosis and management of Mycobacterium tuberculosis-associated uveitis: a review.BMJ Open Ophthalmol. 2021 May 12;6(1):e000663. doi: 10.1136/bmjophth-2020-000663. eCollection 2021. BMJ Open Ophthalmol. 2021. PMID: 34046524 Free PMC article. Review.
-
Clinical application of QuantiFERON-TB Gold in-tube in the diagnosis and treatment of tuberculosis.Eur J Clin Microbiol Infect Dis. 2020 Apr;39(4):607-612. doi: 10.1007/s10096-019-03768-9. Epub 2019 Nov 30. Eur J Clin Microbiol Infect Dis. 2020. PMID: 31786694 Review.
-
QuantiFERON-TB Gold In-tube test for the diagnosis of active and latent tuberculosis in selected health facilities of Addis Ababa, Ethiopia.BMC Res Notes. 2018 May 11;11(1):293. doi: 10.1186/s13104-018-3410-x. BMC Res Notes. 2018. PMID: 29751780 Free PMC article.
References
-
- Organization WH. Global Tuberculosis Control:WHO Report 2013. Genava: WHO; 2013. May.
-
- Organization WH. Global Tuberculosis Control:WHO Report 2012. Genava: WHO; 2012. 258 p.
-
- Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;45(3):322–8. 10.1086/519266 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

