Pentraxin-3 Is a TSH-Inducible Protein in Human Fibrocytes and Orbital Fibroblasts
- PMID: 26287404
- PMCID: PMC4606754
- DOI: 10.1210/en.2015-1399
Pentraxin-3 Is a TSH-Inducible Protein in Human Fibrocytes and Orbital Fibroblasts
Abstract
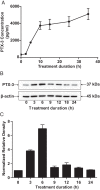
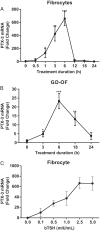
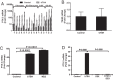
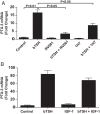
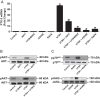
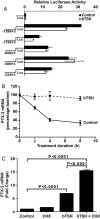
CD34(+) fibrocytes are bone marrow-derived monocyte progenitor cells that traffic to sites of tissue injury and repair. They putatively infiltrate the orbit in thyroid-associated ophthalmopathy where they appear to transition into CD34(+) orbital fibroblasts (OFs) that interact with residential CD34(-) fibroblasts. A unique phenotypic attribute of fibrocytes and CD34(+) OFs is their expression of the functional thyrotropin receptor (TSHR) and other "thyroid-specific" proteins. When activated through TSHR, fibrocytes express a number of cytokines and other inflammatory genes. Here we sought to determine whether pentraxin-3 (PTX-3), an acute-phase protein involved in inflammation and autoimmunity, might be induced by TSH in fibrocytes and OFs. These cells were collected from patients with Graves disease and healthy individuals. PTX-3 mRNA levels were determined by real-time PCR, protein was determined by ELISA and Western blot, and PTX-3 gene promoter activity was assessed with reporter assays. PTX-3 expression was induced by TSH in both cell types, regardless of the health status of the donor and was a consequence of increased steady-state PTX-3 mRNA levels. M22, a TSHR-activating monoclonal antibody, also induced PTX-3. The induction could be attenuated by dexamethasone and by IGF-I receptor-blocking antibodies, teprotumumab and 1H7. TSH effects were mediated through phosphatidylinositol 3-kinase/AKT, mammalian target of rapamycin/p70(s6k), Janus tyrosine kinase 2 pathways, and enhanced PTX-3 mRNA stability. These findings indicate that PTX-3 is a TSH target gene, the expression of which can be induced in fibrocytes and OFs. They suggest that PTX-3 might represent a previously unidentified nexus between the thyroid axis and the mechanisms involved in tissue remodeling.
Figures
Similar articles
-
CD34- Orbital Fibroblasts From Patients With Thyroid-Associated Ophthalmopathy Modulate TNF-α Expression in CD34+ Fibroblasts and Fibrocytes.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2615-2622. doi: 10.1167/iovs.18-23951. Invest Ophthalmol Vis Sci. 2018. PMID: 29847668 Free PMC article.
-
Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production.J Clin Endocrinol Metab. 2012 May;97(5):E740-6. doi: 10.1210/jc.2011-2514. Epub 2012 Mar 7. J Clin Endocrinol Metab. 2012. PMID: 22399514 Free PMC article.
-
Slit2 Modulates the Inflammatory Phenotype of Orbit-Infiltrating Fibrocytes in Graves' Disease.J Immunol. 2018 Jun 15;200(12):3942-3949. doi: 10.4049/jimmunol.1800259. Epub 2018 May 11. J Immunol. 2018. PMID: 29752312 Free PMC article.
-
TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy.Nat Rev Endocrinol. 2015 Mar;11(3):171-81. doi: 10.1038/nrendo.2014.226. Epub 2015 Jan 6. Nat Rev Endocrinol. 2015. PMID: 25560705 Free PMC article. Review.
-
Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy.Best Pract Res Clin Endocrinol Metab. 2012 Jun;26(3):291-302. doi: 10.1016/j.beem.2011.10.002. Best Pract Res Clin Endocrinol Metab. 2012. PMID: 22632366 Free PMC article. Review.
Cited by
-
Potential Therapeutic Activity of Berberine in Thyroid-Associated Ophthalmopathy: Inhibitory Effects on Tissue Remodeling in Orbital Fibroblasts.Invest Ophthalmol Vis Sci. 2022 Sep 1;63(10):6. doi: 10.1167/iovs.63.10.6. Invest Ophthalmol Vis Sci. 2022. PMID: 36094643 Free PMC article.
-
Prenatal Exposure to Lipopolysaccharide Induces PTX3 Expression and Results in Obesity in Mouse Offspring.Inflammation. 2017 Dec;40(6):1847-1861. doi: 10.1007/s10753-017-0626-1. Inflammation. 2017. PMID: 28770376 Free PMC article.
-
The crossroad between autoimmune disorder, tissue remodeling and cancer of the thyroid: The long pentraxin 3 (PTX3).Front Endocrinol (Lausanne). 2023 Mar 21;14:1146017. doi: 10.3389/fendo.2023.1146017. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37025408 Free PMC article. Review.
-
Advances in understanding the role of pentraxin-3 in lung infections.Front Immunol. 2025 Apr 17;16:1575968. doi: 10.3389/fimmu.2025.1575968. eCollection 2025. Front Immunol. 2025. PMID: 40313930 Free PMC article. Review.
-
CD34- Orbital Fibroblasts From Patients With Thyroid-Associated Ophthalmopathy Modulate TNF-α Expression in CD34+ Fibroblasts and Fibrocytes.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2615-2622. doi: 10.1167/iovs.18-23951. Invest Ophthalmol Vis Sci. 2018. PMID: 29847668 Free PMC article.
References
-
- Brent GA. Clinical practice. Graves' disease. N Engl J Med. 2008;358:2594–2605. - PubMed
-
- Regensburg NI, Wiersinga WM, Berendschot TT, Potgieser P, Mourits MP. Do subtypes of graves' orbitopathy exist? Ophthalmology. 2011;118:191–196. - PubMed
-
- Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120:380–386. - PubMed
-
- Smith TJ. Novel aspects of orbital fibroblast pathology. J Endocrinol Invest. 2004;27:246–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

