Leukocyte Populations in Human Preterm and Term Breast Milk Identified by Multicolour Flow Cytometry
- PMID: 26288195
- PMCID: PMC4545889
- DOI: 10.1371/journal.pone.0135580
Leukocyte Populations in Human Preterm and Term Breast Milk Identified by Multicolour Flow Cytometry
Abstract
Background: Extremely preterm infants are highly susceptible to bacterial infections but breast milk provides some protection. It is unknown if leukocyte numbers and subsets in milk differ between term and preterm breast milk. This study serially characterised leukocyte populations in breast milk of mothers of preterm and term infants using multicolour flow cytometry methods for extended differential leukocyte counts in blood.
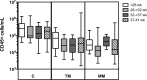
Methods: Sixty mothers of extremely preterm (<28 weeks gestational age), very preterm (28-31 wk), and moderately preterm (32-36 wk), as well as term (37-41 wk) infants were recruited. Colostrum (d2-5), transitional (d8-12) and mature milk (d26-30) samples were collected, cells isolated, and leukocyte subsets analysed using flow cytometry.
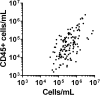
Results: The major CD45+ leukocyte populations circulating in blood were also detectable in breast milk but at different frequencies. Progression of lactation was associated with decreasing CD45+ leukocyte concentration, as well as increases in the relative frequencies of neutrophils and immature granulocytes, and decreases in the relative frequencies of eosinophils, myeloid and B cell precursors, and CD16- monocytes. No differences were observed between preterm and term breast milk in leukocyte concentration, though minor differences between preterm groups in some leukocyte frequencies were observed.
Conclusions: Flow cytometry is a useful tool to identify and quantify leukocyte subsets in breast milk. The stage of lactation is associated with major changes in milk leukocyte composition in this population. Fresh preterm breast milk is not deficient in leukocytes, but shorter gestation may be associated with minor differences in leukocyte subset frequencies in preterm compared to term breast milk.
Conflict of interest statement
Figures


References
-
- Hobbs JR, Davis JA. Serum gamma-G-globulin levels and gestational age in premature babies. Lancet. 1967;1(7493):757–9. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

