The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: a study protocol for three multicentre randomised controlled trials
- PMID: 26289352
- PMCID: PMC4545865
- DOI: 10.1186/s13063-015-0864-1
The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: a study protocol for three multicentre randomised controlled trials
Abstract
Background: Several small trials have suggested that fluoxetine improves neurological recovery from stroke. FOCUS, AFFINITY and EFFECTS are a family of investigator-led, multicentre, parallel group, randomised, placebo-controlled trials that aim to determine whether routine administration of fluoxetine (20 mg daily) for 6 months after acute stroke improves patients' functional outcome.
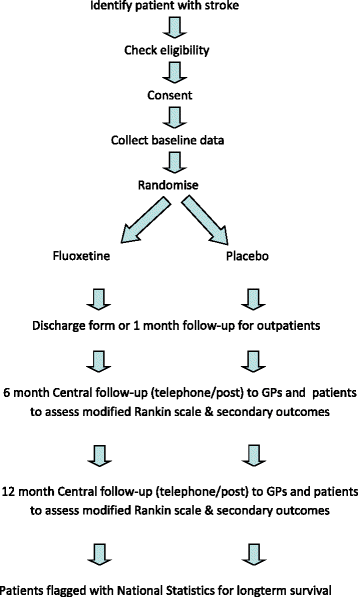
Methods/design: The three trial investigator teams have collaboratively developed a core protocol. Minor variations have been tailored to the national setting in the UK (FOCUS), Australia and New Zealand (AFFINITY) and Sweden (EFFECTS). Each trial is run and funded independently and will report its own results. A prospectively planned individual patient data meta-analysis of all three trials will subsequently provide the most precise estimate of the overall effect of fluoxetine after stroke and establish whether any effects differ between trials and subgroups of patients. The trials include patients ≥18 years old with a clinical diagnosis of stroke, persisting focal neurological deficits at randomisation between 2 and 15 days after stroke onset. Patients are randomised centrally via web-based randomisation systems using a common minimisation algorithm. Patients are allocated fluoxetine 20 mg once daily or matching placebo capsules for 6 months. Our primary outcome measure is the modified Rankin scale (mRS) at 6 months. Secondary outcomes include the Stroke Impact Scale, EuroQol (EQ5D-5 L), the vitality subscale of the Short-Form 36, diagnosis of depression, adherence to medication, adverse events and resource use. Outcomes are collected at 6 and 12 months. The methods of collecting these data are tailored to the national setting. If FOCUS, AFFINITY and EFFECTS combined enrol 6000 participants as planned, they would have 90 % power (alpha 5 %) to detect a common odds ratio of 1.16, equivalent to a 3.7 % absolute difference in percentage with mRS 0-2 (44.0 % to 47.7 %). This is based on an ordinal analysis of mRS adjusted for baseline variables included in the minimisation algorithm.
Discussion: If fluoxetine is safe and effective in promoting functional recovery, it could be rapidly, widely and affordably implemented in routine clinical practice and reduce the burden of disability due to stroke.
Focus: ISRCTN83290762 (23/05/2012), AFFINITY: ACTRN12611000774921 (22/07/2011).
Effects: ISRCTN13020412 (19/12/2014).
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical