NODAL secreted by male germ cells regulates the proliferation and function of human Sertoli cells from obstructive azoospermia and nonobstructive azoospermia patients
- PMID: 26289399
- PMCID: PMC4814958
- DOI: 10.4103/1008-682X.159722
NODAL secreted by male germ cells regulates the proliferation and function of human Sertoli cells from obstructive azoospermia and nonobstructive azoospermia patients
Abstract
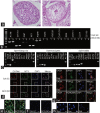
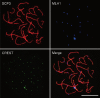
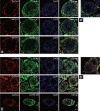
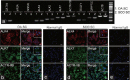
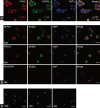
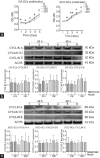
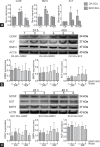
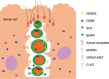
This study was designed to explore the regulatory effects of male germ cell secreting factor NODAL on Sertoli cell fate decisions from obstructive azoospermia (OA) and nonobstructive azoospermia (NOA) patients. Human Sertoli cells and male germ cells were isolated using two-step enzymatic digestion and SATPUT from testes of azoospermia patients. Expression of NODAL and its multiple receptors in human Sertoli cells and male germ cells were characterized by reverse transcription-polymerase chain reaction (RT-PCR) and immunochemistry. Human recombinant NODAL and its receptor inhibitor SB431542 were employed to probe their effect on the proliferation of Sertoli cells using the CCK-8 assay. Quantitative PCR and Western blots were utilized to assess the expression of Sertoli cell functional genes and proteins. NODAL was found to be expressed in male germ cells but not in Sertoli cells, whereas its receptors ALK4, ALK7, and ACTR-IIB were detected in Sertoli cells and germ cells, suggesting that NODAL plays a regulatory role in Sertoli cells and germ cells via a paracrine and autocrine pathway, respectively. Human recombinant NODAL could promote the proliferation of human Sertoli cells. The expression of cell cycle regulators, including CYCLIN A, CYCLIN D1 and CYCLIN E, was not remarkably affected by NODAL signaling. NODAL enhanced the expression of essential growth factors, including GDNF, SCF, and BMP4, whereas SB431542 decreased their levels. There was not homogeneity of genes changes by NODAL treatment in Sertoli cells from OA and Sertoli cell-only syndrome (SCO) patients. Collectively, this study demonstrates that NODAL produced by human male germ cells regulates proliferation and numerous gene expression of Sertoli cells.
Figures


References
-
- Ma M, Yang S, Zhang Z, Li P, Gong Y, et al. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum Reprod. 2013;28:1863–73. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

