Auditory dysfunction in schizophrenia: integrating clinical and basic features
- PMID: 26289573
- PMCID: PMC4692466
- DOI: 10.1038/nrn4002
Auditory dysfunction in schizophrenia: integrating clinical and basic features
Abstract
Schizophrenia is a complex neuropsychiatric disorder that is associated with persistent psychosocial disability in affected individuals. Although studies of schizophrenia have traditionally focused on deficits in higher-order processes such as working memory and executive function, there is an increasing realization that, in this disorder, deficits can be found throughout the cortex and are manifest even at the level of early sensory processing. These deficits are highly amenable to translational investigation and represent potential novel targets for clinical intervention. Deficits, moreover, have been linked to specific structural abnormalities in post-mortem auditory cortex tissue from individuals with schizophrenia, providing unique insights into underlying pathophysiological mechanisms.
Conflict of interest statement
The authors declare competing interests: see Web version for details.
Figures

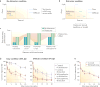

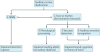
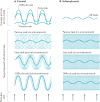

References
-
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. This study illustrates the developmental course of neural changes in schizophrenia. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

